Inactivation of the constitutively active ghrelin receptor attenuates limbic seizure activity in rodents
- PMID: 22669710
- PMCID: PMC3441926
- DOI: 10.1007/s13311-012-0125-x
Inactivation of the constitutively active ghrelin receptor attenuates limbic seizure activity in rodents
Abstract
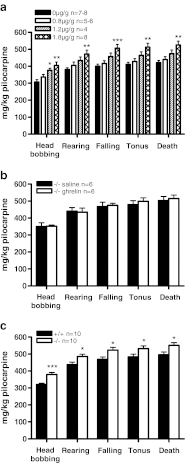
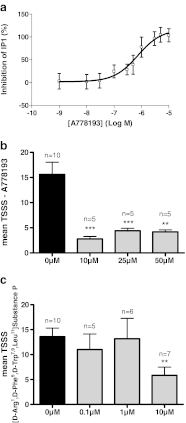
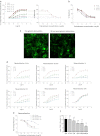
Ghrelin is a pleiotropic neuropeptide that has been recently implicated in epilepsy. Animal studies performed to date indicate that ghrelin has anticonvulsant properties; however, its mechanism of anticonvulsant action is unknown. Here we show that the anticonvulsant effects of ghrelin are mediated via the growth hormone secretagogue receptor (GHSR). To our surprise, however, we found that the GHSR knockout mice had a higher seizure threshold than their wild-type littermates when treated with pilocarpine. Using both in vivo and in vitro models, we further discovered that inverse agonism and desensitization/internalization of the GHSR attenuate limbic seizures in rats and epileptiform activity in hippocampal slices. This constitutes a novel mechanism of anticonvulsant action, whereby an endogenous agonist reduces the activity of a constitutively active receptor.
Figures

References
-
- Angelidis G, Valotassiou V, Georgoulias P. Current and potential roles of ghrelin in clinical practice. J Endocrinol Invest. 2010;33:823–838. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

