Plant peroxisomes: biogenesis and function
- PMID: 22669882
- PMCID: PMC3406917
- DOI: 10.1105/tpc.112.096586
Plant peroxisomes: biogenesis and function
Abstract
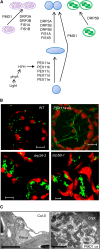
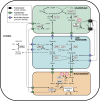
Peroxisomes are eukaryotic organelles that are highly dynamic both in morphology and metabolism. Plant peroxisomes are involved in numerous processes, including primary and secondary metabolism, development, and responses to abiotic and biotic stresses. Considerable progress has been made in the identification of factors involved in peroxisomal biogenesis, revealing mechanisms that are both shared with and diverged from non-plant systems. Furthermore, recent advances have begun to reveal an unexpectedly large plant peroxisomal proteome and have increased our understanding of metabolic pathways in peroxisomes. Coordination of the biosynthesis, import, biochemical activity, and degradation of peroxisomal proteins allows for highly dynamic responses of peroxisomal metabolism to meet the needs of a plant. Knowledge gained from plant peroxisomal research will be instrumental to fully understanding the organelle's dynamic behavior and defining peroxisomal metabolic networks, thus allowing the development of molecular strategies for rational engineering of plant metabolism, biomass production, stress tolerance, and pathogen defense.
Figures



References
-
- Acosta I.F., Farmer E.E. (2010). Jasmonates. The Arabidopsis Book 8: e0129. doi/10.1199/tab.0129
-
- Adham A.R., Zolman B.K., Millius A., Bartel B. (2005). Mutations in Arabidopsis acyl-CoA oxidase genes reveal distinct and overlapping roles in beta-oxidation. Plant J. 41: 859–874 - PubMed
-
- Afitlhile M.M., Fukushige H., Nishimura M., Hildebrand D.F. (2005). A defect in glyoxysomal fatty acid beta-oxidation reduces jasmonic acid accumulation in Arabidopsis. Plant Physiol. Biochem. 43: 603–609 - PubMed
-
- Anand P., Kwak Y., Simha R., Donaldson R.P. (2009). Hydrogen peroxide induced oxidation of peroxisomal malate synthase and catalase. Arch. Biochem. Biophys. 491: 25–31 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

