Longitudinal evaluation of MPIO-labeled stem cell biodistribution in glioblastoma using high resolution and contrast-enhanced MR imaging at 14.1 tesla
- PMID: 22670012
- PMCID: PMC3408258
- DOI: 10.1093/neuonc/nos126
Longitudinal evaluation of MPIO-labeled stem cell biodistribution in glioblastoma using high resolution and contrast-enhanced MR imaging at 14.1 tesla
Abstract
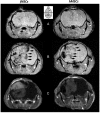
To optimize the development of stem cell (SC)-based therapies for the treatment of glioblastoma (GBM), we compared the pathotropism of 2 SC sources, human mesenchymal stem cells (hMSCs) and fetal neural stem cells (fNSCs), toward 2 orthotopic GBM models, circumscribed U87vIII and highly infiltrative GBM26. High resolution and contrast-enhanced (CE) magnetic resonance imaging (MRI) were performed at 14.1 Tesla to longitudinally monitor the in vivo location of hMSCs and fNSCs labeled with the same amount of micron-size particles of iron oxide (MPIO). To assess pathotropism, SCs were injected in the contralateral hemisphere of U87vIII tumor-bearing mice. Both MPIO-labeled SC types exhibited tropism to tumors, first localizing at the tumor edges, then in the tumor masses. MPIO-labeled hMSCs and fNSCs were also injected intratumorally in mice with U87vIII or GBM26 tumors to assess their biodistribution. Both SC types distributed throughout the tumor in both GBM models. Of interest, in the U87vIII model, areas of hyposignal colocalized first with the enhancing regions (ie, regions of high vascular permeability), consistent with SC tropism to vascular endothelial growth factor. In the GBM26 model, no rim of hyposignal was observed, consistent with the infiltrative nature of this tumor. Quantitative analysis of the index of dispersion confirmed that both MPIO-labeled SC types longitudinally distribute inside the tumor masses after intratumoral injection. Histological studies confirmed the MRI results. In summary, our results indicate that hMSCs and fNSCs exhibit similar properties regarding tumor tropism and intratumoral dissemination, highlighting the potential of these 2 SC sources as adequate candidates for SC-based therapies.
Figures
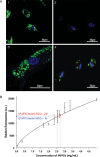



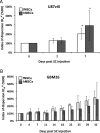
 as a percentage of
as a percentage of  plotted for each imaging day post intratumoral injection of MPIO-labeled hMSCs (grey) and MPIO-labeled fNSCs (white) in (A) U87vIII and (B) GBM26 tumor-bearing mice. A significant increase of the index of dispersion
plotted for each imaging day post intratumoral injection of MPIO-labeled hMSCs (grey) and MPIO-labeled fNSCs (white) in (A) U87vIII and (B) GBM26 tumor-bearing mice. A significant increase of the index of dispersion  was observed for both SC sources between day 0 and day 7 for the U87vIII tumor type (*P < .05), and no significant differences were found between cell types. In the GBM26 model, an increase in
was observed for both SC sources between day 0 and day 7 for the U87vIII tumor type (*P < .05), and no significant differences were found between cell types. In the GBM26 model, an increase in  was also observed between day 0 and day 32 for both SC sources.
was also observed between day 0 and day 32 for both SC sources.

References
-
- CBRTUS. Statistical report - Primary brain and central nervous system tumors diagnosed in the United states in 2004–2006. 2010. http://seer.cancer.gov/ - PMC - PubMed
-
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi:10.1007/s00401-007-0243-4. - DOI - PMC - PubMed
-
- Clarke J, Butowski N, Chang S. Recent advances in therapy for glioblastoma. Arch Neurol. 2010;67(3):279–283. doi:10.1001/archneurol.2010.5. - DOI - PubMed
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi:10.1016/S1470-2045(09)70025-7. - DOI - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi:10.1056/NEJMoa043330. - DOI - PubMed

