Comparison of international breast cancer guidelines: are we globally consistent? cancer guideline AGREEment
- PMID: 22670108
- PMCID: PMC3364779
- DOI: 10.3747/co.19.930
Comparison of international breast cancer guidelines: are we globally consistent? cancer guideline AGREEment
Abstract
Background: Evidence-based guidelines are used in health care systems throughout the world to aid in treatment decisions and to ensure quality and consistency in patient care. In breast oncology, guidelines for care are published by several internationally recognized organizations, including those from the United States, Canada, and the United Kingdom. The present study compared clinical breast cancer guidelines from the American Society of Clinical Oncology (ASCO, United States), Cancer Care Ontario (CCO, Canada), and the National Institute for Health and Clinical Excellence (NICE, United Kingdom) to determine the quality and consistency of content across international organizations.
Methods: We searched for breast cancer guidelines published by ASCO, CCO, and NICE. Guidelines on the same theme were identified across organizations and appraised by 4 independent reviewers using the Appraisal of Guidelines for Research and Evaluation (AGREE) instrument. Content of each guideline was also scored for consistency in overall recommendations across organizations and for consistency in cited evidence.
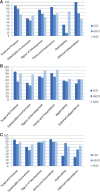
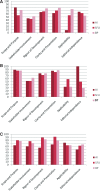
Results: The quality of breast cancer guidelines produced by the targeted organizations was consistently good in the areas of Scope and Purpose, Rigor of Development, and Clarity and Presentation, but variable in the domains of Stakeholder Involvement, Applicability, and Editorial Independence. The content of the guidelines varied slightly in the strength of their recommendations.
Conclusions: Our review demonstrated consistency in quality and content for breast cancer practice guidelines published by various organizations. Future guidelines developed by these organizations should focus on how to implement and measure uptake of a guideline.
Keywords: Breast cancer; agree instrument; practice guidelines; quality of care.
Figures
Similar articles
-
Quality assessment of clinical practice guidelines on psychological distress of cancer patients using the AGREE II instrument.Front Oncol. 2022 Aug 9;12:942219. doi: 10.3389/fonc.2022.942219. eCollection 2022. Front Oncol. 2022. PMID: 36016612 Free PMC article.
-
An independent AGREE evaluation of the Occupational Medicine Practice Guidelines.Spine J. 2006 Jan-Feb;6(1):72-7. doi: 10.1016/j.spinee.2005.06.012. Spine J. 2006. PMID: 16413451
-
Clinical practice guidelines on prostate cancer: a critical appraisal.J Urol. 2015 Apr;193(4):1153-8. doi: 10.1016/j.juro.2014.10.105. Epub 2014 Nov 4. J Urol. 2015. PMID: 25451831 Review.
-
World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) Guidelines update - IV - A quality appraisal with the AGREE II instrument.World Allergy Organ J. 2022 Mar 2;15(2):100613. doi: 10.1016/j.waojou.2021.100613. eCollection 2022 Feb. World Allergy Organ J. 2022. PMID: 36091188 Free PMC article.
-
A critical appraisal of the North American Spine Society guidelines with the Appraisal of Guidelines for Research and Evaluation II instrument.Spine J. 2015 Apr 1;15(4):777-81. doi: 10.1016/j.spinee.2015.01.012. Epub 2015 Jan 19. Spine J. 2015. PMID: 25614152
Cited by
-
What are the appropriate thresholds for High Quality Performance Indicators for breast surgery in Australia and New Zealand?Breast. 2020 Jun;51:94-101. doi: 10.1016/j.breast.2020.01.007. Epub 2020 Jan 30. Breast. 2020. PMID: 32252005 Free PMC article.
-
Quality and reporting of clinical guidelines for breast cancer treatment: A systematic review.Breast. 2020 Oct;53:201-211. doi: 10.1016/j.breast.2020.07.011. Epub 2020 Aug 10. Breast. 2020. PMID: 32858405 Free PMC article.
-
Staging Investigations in Breast Cancer: Collective Opinion of UK Breast Surgeons.Int J Breast Cancer. 2013;2013:506172. doi: 10.1155/2013/506172. Epub 2013 Nov 20. Int J Breast Cancer. 2013. PMID: 24349790 Free PMC article.
-
The development of guideline implementation tools: a qualitative study.CMAJ Open. 2015 Jan 13;3(1):E127-33. doi: 10.9778/cmajo.20140064. eCollection 2015 Jan-Mar. CMAJ Open. 2015. PMID: 25844365 Free PMC article.
-
A quality evaluation of the clinical practice guidelines on breast cancer using the RIGHT checklist.Ann Transl Med. 2021 Jul;9(14):1174. doi: 10.21037/atm-21-2884. Ann Transl Med. 2021. PMID: 34430615 Free PMC article.
References
-
- Public Health Agency of Canada (phac) Home > Chronic Diseases > Cancer > Breast Cancer [Web page] Ottawa, ON: PHAC; 2009. [Available at: http://www.phac-aspc.gc.ca/cd-mc/cancer/breast_cancer-cancer_du_sein-eng...; cited May 15, 2011]
-
- Altekruse SF, Kosary CL, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: US National Cancer Institute; 2010. [Available for download at: http://seer.cancer.gov/csr/1975_2007; cited April 19, 2012]
-
- Cancer Research UK . Home > CancerStats > Types of cancer > Breast Cancer > Incidence [Web page, see “Lifetime Risk” subsection] London, U.K.: Cancer Research UK; 2010. [Available at: http://info.cancerresearchuk.org/cancerstats/types/breast/incidence/#Lif...; cited January 7, 2009]
LinkOut - more resources
Full Text Sources

