Drug transporters in spermatogenesis: A re-evaluation of recent data on P-glycoprotein
- PMID: 22670215
- PMCID: PMC3364793
- DOI: 10.4161/spmg.20507
Drug transporters in spermatogenesis: A re-evaluation of recent data on P-glycoprotein
Abstract
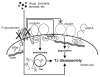
Drug transporters are integral membrane proteins expressed by a variety of organs, including the liver, kidney, small intestine and testis, and they are generally known to mediate drug or xenobiotic transport into and out of cells. Previous studies have also reported the presence of several drug transporters at blood-tissue barriers where they are thought to protect organs from harmful agents. In this editorial, we briefly discuss and re-evaluate recent findings that show P-glycoprotein, an efflux pump, to function at the blood-testis barrier. We also put forth a mechanistic model, hoping this information will form a strong basis for future studies.
Figures
Similar articles
-
Emerging role for drug transporters at the blood-testis barrier.Trends Pharmacol Sci. 2011 Feb;32(2):99-106. doi: 10.1016/j.tips.2010.11.007. Epub 2010 Dec 17. Trends Pharmacol Sci. 2011. PMID: 21168226 Free PMC article. Review.
-
Drug transporters, the blood-testis barrier, and spermatogenesis.J Endocrinol. 2011 Mar;208(3):207-23. doi: 10.1677/JOE-10-0363. Epub 2010 Dec 6. J Endocrinol. 2011. PMID: 21134990 Free PMC article. Review.
-
Membrane transporter proteins: a challenge for CNS drug development.Dialogues Clin Neurosci. 2006;8(3):311-21. doi: 10.31887/DCNS.2006.8.3/fgirardin. Dialogues Clin Neurosci. 2006. PMID: 17117613 Free PMC article. Review.
-
Regulation of drug transporters in the testis by environmental toxicant cadmium, steroids and cytokines.Spermatogenesis. 2012 Oct 1;2(4):285-293. doi: 10.4161/spmg.22536. Spermatogenesis. 2012. PMID: 23248770 Free PMC article.
-
Transport of organic cations across the blood-testis barrier.Mol Pharm. 2007 Jul-Aug;4(4):600-7. doi: 10.1021/mp070023l. Epub 2007 Jul 7. Mol Pharm. 2007. PMID: 17616214
Cited by
-
Signaling pathways regulating blood-tissue barriers - Lesson from the testis.Biochim Biophys Acta Biomembr. 2018 Jan;1860(1):141-153. doi: 10.1016/j.bbamem.2017.04.020. Epub 2017 Apr 25. Biochim Biophys Acta Biomembr. 2018. PMID: 28450047 Free PMC article. Review.
-
Role of MDR1 Gene Polymorphisms in Human Male Infertility: A Meta-Analysis.Am J Mens Health. 2023 Mar-Apr;17(2):15579883231166645. doi: 10.1177/15579883231166645. Am J Mens Health. 2023. PMID: 37081725 Free PMC article.
-
Fluoxetine Mitigates Human Sperm Quality by Disrupting the Antioxidant Defense System and Altering the Expression of Apoptosis-Related Genes: An In Vitro Study.Reprod Sci. 2025 Feb;32(2):326-342. doi: 10.1007/s43032-024-01760-z. Epub 2025 Jan 9. Reprod Sci. 2025. PMID: 39789370
References
Grants and funding
LinkOut - more resources
Full Text Sources
