Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome
- PMID: 22670904
- PMCID: PMC4362529
- DOI: 10.1056/NEJMoa1113538
Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome
Abstract
Background: Dysregulated hedgehog signaling is the pivotal molecular abnormality underlying basal-cell carcinomas. Vismodegib is a new orally administered hedgehog-pathway inhibitor that produces objective responses in locally advanced and metastatic basal-cell carcinomas.
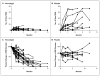
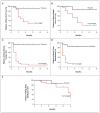
Methods: We tested the anti-basal-cell carcinoma efficacy of vismodegib in a randomized, double-blind, placebo-controlled trial in patients with the basal-cell nevus syndrome at three clinical centers from September 2009 through January 2011. The primary end point was reduction in the incidence of new basal-cell carcinomas that were eligible for surgical resection (surgically eligible) with vismodegib versus placebo after 3 months; secondary end points included reduction in the size of existing basal-cell carcinomas.
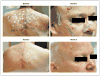
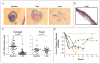
Results: In 41 patients followed for a mean of 8 months (range, 1 to 15) after enrollment, the per-patient rate of new surgically eligible basal-cell carcinomas was lower with vismodegib than with placebo (2 vs. 29 cases per group per year, P<0.001), as was the size (percent change from baseline in the sum of the longest diameter) of existing clinically significant basal-cell carcinomas (-65% vs. -11%, P=0.003). In some patients, all basal-cell carcinomas clinically regressed. No tumors progressed during treatment with vismodegib. Patients receiving vismodegib routinely had grade 1 or 2 adverse events of loss of taste, muscle cramps, hair loss, and weight loss. Overall, 54% of patients (14 of 26) receiving vismodegib discontinued drug treatment owing to adverse events. At 1 month, vismodegib use had reduced the hedgehog target-gene expression by basal-cell carcinoma by 90% (P<0.001) and diminished tumor-cell proliferation, but apoptosis was not affected. No residual basal-cell carcinoma was detectable in 83% of biopsy samples taken from sites of clinically regressed basal-cell carcinomas.
Conclusions: Vismodegib reduces the basal-cell carcinoma tumor burden and blocks growth of new basal-cell carcinomas in patients with the basal-cell nevus syndrome. The adverse events associated with treatment led to discontinuation in over half of treated patients. (Funded by Genentech and others; ClinicalTrials.gov number, NCT00957229.).
Figures
Comment in
-
Oral hedgehog-pathway inhibitors for basal-cell carcinoma.N Engl J Med. 2012 Jun 7;366(23):2225-6. doi: 10.1056/NEJMe1202170. N Engl J Med. 2012. PMID: 22670909 No abstract available.
-
Vismodegib in advanced basal-cell carcinoma.N Engl J Med. 2012 Sep 6;367(10):970; author reply 970-1. doi: 10.1056/NEJMc1208003. N Engl J Med. 2012. PMID: 22931271 No abstract available.
References
-
- Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774–8. - PubMed
-
- Gorlin RJ. Nevoid basal cell carcinoma syndrome. Medicine (Baltimore) 1987;66:98–113. - PubMed
-
- Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–71. - PubMed
-
- Hahn H, Wicking C, Zaphiropoulous PG, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–51. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
