Protection of dopaminergic cells by urate requires its accumulation in astrocytes
- PMID: 22671773
- PMCID: PMC3438313
- DOI: 10.1111/j.1471-4159.2012.07820.x
Protection of dopaminergic cells by urate requires its accumulation in astrocytes
Abstract
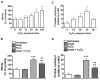
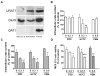
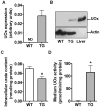
Urate is the end product of purine metabolism and a major antioxidant circulating in humans. Recent data link higher levels of urate with a reduced risk of developing Parkinson's disease and with a slower rate of its progression. In this study, we investigated the role of astrocytes in urate-induced protection of dopaminergic cells in a cellular model of Parkinson's disease. In mixed cultures of dopaminergic cells and astrocytes oxidative stress-induced cell death and protein damage were reduced by urate. By contrast, urate was not protective in pure dopaminergic cell cultures. Physical contact between dopaminergic cells and astrocytes was not required for astrocyte-dependent rescue as shown by conditioned medium experiments. Urate accumulation in dopaminergic cells and astrocytes was blocked by pharmacological inhibitors of urate transporters expressed differentially in these cells. The ability of a urate transport blocker to prevent urate accumulation into astroglial (but not dopaminergic) cells predicted its ability to prevent dopaminergic cell death. Transgenic expression of uricase reduced urate accumulation in astrocytes and attenuated the protective influence of urate on dopaminergic cells. These data indicate that urate might act within astrocytes to trigger release of molecule(s) that are protective for dopaminergic cells.
© 2012 The Authors. Journal of Neurochemistry © 2012 International Society for Neurochemistry.
Figures

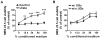
Similar articles
-
Protection by inosine in a cellular model of Parkinson's disease.Neuroscience. 2014 Aug 22;274:242-9. doi: 10.1016/j.neuroscience.2014.05.038. Epub 2014 May 29. Neuroscience. 2014. PMID: 24880154 Free PMC article.
-
Urate and its transgenic depletion modulate neuronal vulnerability in a cellular model of Parkinson's disease.PLoS One. 2012;7(5):e37331. doi: 10.1371/journal.pone.0037331. Epub 2012 May 14. PLoS One. 2012. PMID: 22606360 Free PMC article.
-
The protective effect of astrocyte-derived 14,15-epoxyeicosatrienoic acid on hydrogen peroxide-induced cell injury in astrocyte-dopaminergic neuronal cell line co-culture.Neuroscience. 2012 Oct 25;223:68-76. doi: 10.1016/j.neuroscience.2012.07.045. Epub 2012 Jul 31. Neuroscience. 2012. PMID: 22863680 Free PMC article.
-
Targeting urate to reduce oxidative stress in Parkinson disease.Exp Neurol. 2017 Dec;298(Pt B):210-224. doi: 10.1016/j.expneurol.2017.06.017. Epub 2017 Jun 13. Exp Neurol. 2017. PMID: 28622913 Free PMC article. Review.
-
Towards the physiological function of uric acid.Free Radic Biol Med. 1993 Jun;14(6):615-31. doi: 10.1016/0891-5849(93)90143-i. Free Radic Biol Med. 1993. PMID: 8325534 Review.
Cited by
-
Urate-induced immune programming: Consequences for gouty arthritis and hyperuricemia.Immunol Rev. 2020 Mar;294(1):92-105. doi: 10.1111/imr.12833. Epub 2019 Dec 19. Immunol Rev. 2020. PMID: 31853991 Free PMC article. Review.
-
The Role of Oxidative Stress in Parkinson's Disease.Antioxidants (Basel). 2020 Jul 8;9(7):597. doi: 10.3390/antiox9070597. Antioxidants (Basel). 2020. PMID: 32650609 Free PMC article. Review.
-
Ethyl Pyruvate Increases Post-Ischemic Levels of Mitochondrial Energy Metabolites: A 13C-Labeled Cerebral Microdialysis Study.Metabolites. 2020 Jul 13;10(7):287. doi: 10.3390/metabo10070287. Metabolites. 2020. PMID: 32668656 Free PMC article.
-
Interaction Between ITM2B and GLUT9 Links Urate Transport to Neurodegenerative Disorders.Front Physiol. 2019 Oct 22;10:1323. doi: 10.3389/fphys.2019.01323. eCollection 2019. Front Physiol. 2019. PMID: 31695625 Free PMC article.
-
Urate levels predict survival in amyotrophic lateral sclerosis: Analysis of the expanded Pooled Resource Open-Access ALS clinical trials database.Muscle Nerve. 2018 Mar;57(3):430-434. doi: 10.1002/mus.25950. Epub 2017 Sep 21. Muscle Nerve. 2018. PMID: 28857199 Free PMC article.
References
-
- Alebouyeh M, Takeda M, Onozato ML, Tojo A, Noshiro R, Hasannejad H, Inatomi J, Narikawa S, Huang XL, Khamdang S, Anzai N, Endou H. Expression of human organic anion transporters in the choroid plexus and their interactions with neurotransmitter metabolites. J Pharmacol Sci. 2003;93:430–436. - PubMed
-
- Bahn A, Ljubojevic M, Lorenz H, Schultz C, Ghebremedhin E, Ugele B, Sabolic I, Burckhardt G, Hagos Y. Murine renal organic anion transporters mOAT1 and mOAT3 facilitate the transport of neuroactive tryptophan metabolites. Am J Physiol Cell Physiol. 2005;289:C1075–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

