In vitro metabolism of piperaquine is primarily mediated by CYP3A4
- PMID: 22671777
- PMCID: PMC5087332
- DOI: 10.3109/00498254.2012.693972
In vitro metabolism of piperaquine is primarily mediated by CYP3A4
Abstract
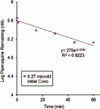
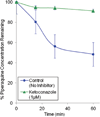
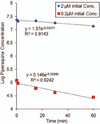
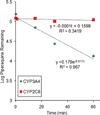
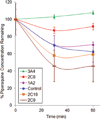
Piperaquine (PQ) is part of a first-line treatment regimen for Plasmodium falciparum malaria recommended by the World Health Organization (WHO). We aimed to determine the major metabolic pathway(s) of PQ in vitro. A reliable, validated tandem mass spectrometry method was developed. Concentrations of PQ were measured after incubation with both human liver microsomes (HLMs) and expressed cytochrome P450 enzymes (P450s). In pooled HLMs, incubations with an initial PQ concentration of 0.3 µM resulted in a 34.8 ± 4.9% loss of substrate over 60 min, corresponding to a turnover rate of 0.009 min(-1) (r(2) = 0.9223). Miconazole, at nonspecific P450 inhibitory concentrations, resulted in almost complete inhibition of PQ metabolism. The greatest inhibition was demonstrated with selective CYP3A4 (100%) and CYP2C8 (66%) inhibitors. Using a mixture of recombinant P450 enzymes, turnover for PQ metabolism was estimated as 0.0099 min(-1); recombinant CYP3A4 had a higher metabolic rate (0.017 min(-1)) than recombinant CYP2C8 (p < .0001). Inhibition of CYP3A4-mediated PQ loss was greatest using the selective inhibitor ketoconazole (9.1 ± 3.5% loss with ketoconazole vs 60.7 ± 5.9% with no inhibitor, p < .0001). In summary, the extent of inhibition of in vitro metabolism with ketoconazole (83%) denotes that PQ appears to be primarily catalyzed by CYP3A4. Further studies to support these findings through the identification and characterization of PQ metabolites are planned.
Figures
Similar articles
-
Drug-drug Interaction between Losartan and Paclitaxel in Human Liver Microsomes with Different CYP2C8 Genotypes.Basic Clin Pharmacol Toxicol. 2015 Jun;116(6):493-8. doi: 10.1111/bcpt.12355. Epub 2014 Dec 23. Basic Clin Pharmacol Toxicol. 2015. PMID: 25424246
-
Autoinhibition of CYP3A4 leads to important role of CYP2C8 in imatinib metabolism: variability in CYP2C8 activity may alter plasma concentrations and response.Drug Metab Dispos. 2013 Jan;41(1):50-9. doi: 10.1124/dmd.112.048017. Epub 2012 Oct 1. Drug Metab Dispos. 2013. PMID: 23028140
-
CYP2C8- and CYP3A-mediated C-demethylation of (3-{[(4-tert-butylbenzyl)-(pyridine-3-sulfonyl)-amino]-methyl}-phenoxy)-acetic acid (CP-533,536), an EP2 receptor-selective prostaglandin E2 agonist: characterization of metabolites by high-resolution liquid chromatography-tandem mass spectrometry and liquid chromatography/mass spectrometry-nuclear magnetic resonance.Drug Metab Dispos. 2008 Oct;36(10):2093-103. doi: 10.1124/dmd.108.022897. Epub 2008 Jul 24. Drug Metab Dispos. 2008. PMID: 18653741
-
Metabolism of repaglinide by CYP2C8 and CYP3A4 in vitro: effect of fibrates and rifampicin.Basic Clin Pharmacol Toxicol. 2005 Oct;97(4):249-56. doi: 10.1111/j.1742-7843.2005.pto_157.x. Basic Clin Pharmacol Toxicol. 2005. PMID: 16176562
-
Estimation of the Contribution of CYP2C8 and CYP3A4 in Repaglinide Metabolism by Human Liver Microsomes Under Various Buffer Conditions.J Pharm Sci. 2017 Sep;106(9):2847-2852. doi: 10.1016/j.xphs.2017.02.013. Epub 2017 Feb 24. J Pharm Sci. 2017. PMID: 28238899
Cited by
-
Effect of Dihydroartemisinin-Piperaquine on the Pharmacokinetics of Praziquantel for Treatment of Schistosoma mansoni Infection.Pharmaceuticals (Basel). 2021 Apr 23;14(5):400. doi: 10.3390/ph14050400. Pharmaceuticals (Basel). 2021. PMID: 33922522 Free PMC article.
-
Reduced Exposure to Piperaquine, Compared to Adults, in Young Children Receiving Dihydroartemisinin-Piperaquine as Malaria Chemoprevention.Clin Pharmacol Ther. 2019 Dec;106(6):1310-1318. doi: 10.1002/cpt.1534. Epub 2019 Jul 22. Clin Pharmacol Ther. 2019. PMID: 31173649 Free PMC article.
-
Metabolism of Piperaquine to Its Antiplasmodial Metabolites and Their Pharmacokinetic Profiles in Healthy Volunteers.Antimicrob Agents Chemother. 2018 Jul 27;62(8):e00260-18. doi: 10.1128/AAC.00260-18. Print 2018 Aug. Antimicrob Agents Chemother. 2018. PMID: 29784841 Free PMC article.
-
In vitro interaction of lumefantrine and piperaquine by atorvastatin against Plasmodium falciparum.Malar J. 2014 May 25;13:189. doi: 10.1186/1475-2875-13-189. Malar J. 2014. PMID: 24886347 Free PMC article.
-
Post-licensure safety evaluation of dihydroartemisinin piperaquine in the three major ecological zones across Ghana.PLoS One. 2017 Mar 30;12(3):e0174503. doi: 10.1371/journal.pone.0174503. eCollection 2017. PLoS One. 2017. PMID: 28358871 Free PMC article.
References
-
- Bjornsson TD, Callaghan JT, Einolf HJ, Fischer V, Gan L, Grimm S, Kao J, King SP, Miwa G, Ni L, Kumar G, McLeod J, Obach RS, Roberts S, Roe A, Shah A, Snikeris F, Sullivan JT, Tweedie D, Vega JM, Walsh J, Wrighton SA Pharmaceutical Research and Manufacturers of America (PhRMA) Drug Metabolism/Clinical Pharmacology Technical Working Group; FDA Center for Drug Evaluation and Research (CDER) The conduct of in vitro and in vivo drug-drug interaction studies: A Pharmaceutical Research and Manufacturers of America (PhRMA) perspective. Drug Metab Dispos. 2003;31:815–832. - PubMed
-
- Davis TM, Hung TY, Sim IK, Karunajeewa HA, Ilett KF. Piperaquine: A resurgent antimalarial drug. Drugs. 2005;65:75–87. - PubMed
-
- Donahue SR, Flockhart DA, Abernethy DR, Ko JW. Ticlopidine inhibition of phenytoin metabolism mediated by potent inhibition of CYP2C19. Clin Pharmacol Ther. 1997;62:572–577. - PubMed
-
- Food and Drug Administration. Guidance for industry: Bioanalytical method validation. [Accessed on: 28 July, 2009];2001 Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati....
-
- German P, Parikh S, Lawrence J, Dorsey G, Rosenthal PJ, Havlir D, Charlebois E, Hanpithakpong W, Lindegardh N, Aweeka FT. Lopinavir/ritonavir affects pharmacokinetic exposure of artemether/lumefantrine in HIV-uninfected healthy volunteers. J Acquir Immune Defic Syndr. 2009;51:424–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
