C-H bond functionalization via hydride transfer: formation of α-arylated piperidines and 1,2,3,4-tetrahydroisoquinolines via stereoselective intramolecular amination of benzylic C-H bonds
- PMID: 22672002
- PMCID: PMC3433405
- DOI: 10.1021/jo300635m
C-H bond functionalization via hydride transfer: formation of α-arylated piperidines and 1,2,3,4-tetrahydroisoquinolines via stereoselective intramolecular amination of benzylic C-H bonds
Abstract
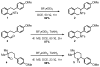
We here report a study of the intramolecular amination of sp(3) C-H bonds via the hydride transfer cyclization of N-tosylimines (HT-amination). In this transformation, 5-aryl aldehydes are subjected to N-toluenesulfonamide in the presence of BF(3)·OEt(2) to effect imine formation and HT-cyclization, leading to 2-arylpiperidines and 3-aryl-1,2,3,4-tetrahydroisoquinolines in a one-pot procedure. We examined the reactivity of a range of aldehyde substrates as a function of their conformational flexibility. Substrates of higher conformational rigidity were more reactive, giving higher yields of the desired products. However, a single substituent on the alkyl chain linking the N-tosylimine and the benzylic sp(3) C-H bonds was sufficient for HT-cyclization to occur. In addition, an examination of various arenes revealed that the electronic character of the hydridic C-H bonds dramatically affects the efficiency of the reaction. We also found that this transformation is highly stereoselective; 2-substituted aldehydes yield cis-2,5-disubstituted piperidines, while 3-substituted aldehydes afford trans-2,4-disubstituted piperidines. The stereoselectivity is a consequence of thermodynamic control. The pseudoallylic strain between the arene and tosyl group on the piperidine ring is proposed to rationalize the greater stability of the isomer with the aryl ring in the axial position. This preferential placement of the arene is proposed to affect the observed stereoselectivity.
Figures
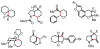
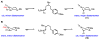
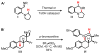


Similar articles
-
Accessing 2-substituted piperidine iminosugars by organometallic addition/intramolecular reductive amination: aldehyde vs. nitrone route.Org Biomol Chem. 2017 Nov 7;15(43):9121-9126. doi: 10.1039/c7ob01848g. Org Biomol Chem. 2017. PMID: 29051928
-
Synthesis of 3,4-disubstituted piperidines by carbonyl ene and prins cyclizations: switching between kinetic and thermodynamic control with Brønsted and Lewis acid catalysts.J Org Chem. 2006 Mar 17;71(6):2460-71. doi: 10.1021/jo052532+. J Org Chem. 2006. PMID: 16526798
-
Stereodivergent Intramolecular C(sp(3))-H Functionalization of Azavinyl Carbenes: Synthesis of Saturated Heterocycles and Fused N-Heterotricycles.J Am Chem Soc. 2015 Jul 8;137(26):8368-71. doi: 10.1021/jacs.5b04295. Epub 2015 Jun 29. J Am Chem Soc. 2015. PMID: 26096731
-
[Synthetic studies on natural products with aromatic nitrogen heterocycles based on development of the methods for the formation of aryl carbon-nitrogen bond].Yakugaku Zasshi. 2013;133(10):1065-78. doi: 10.1248/yakushi.13-00189. Yakugaku Zasshi. 2013. PMID: 24088350 Review. Japanese.
-
Selective functionalisation of saturated C-H bonds with metalloporphyrin catalysts.Chem Soc Rev. 2011 Apr;40(4):1950-75. doi: 10.1039/c0cs00142b. Epub 2011 Mar 9. Chem Soc Rev. 2011. PMID: 21387046 Review.
Cited by
-
Lewis acid-catalyzed redox-neutral amination of 2-(3-pyrroline-1-yl)benzaldehydes via intramolecular [1,5]-hydride shift/isomerization reaction.Beilstein J Org Chem. 2014 Dec 5;10:2892-6. doi: 10.3762/bjoc.10.306. eCollection 2014. Beilstein J Org Chem. 2014. PMID: 25550755 Free PMC article.
-
Highly diastereoselective synthesis of tricyclic fused-pyrans by sequential hydride shift mediated double C(sp3)-H bond functionalization.Chem Sci. 2018 Jun 25;9(37):7327-7331. doi: 10.1039/c8sc02103a. eCollection 2018 Oct 7. Chem Sci. 2018. PMID: 30542535 Free PMC article.
-
Intramolecular Redox-Mannich Reactions: Facile Access to the Tetrahydroprotoberberine Core.Chemistry. 2015 Sep 7;21(37):12908-13. doi: 10.1002/chem.201501667. Epub 2015 Jul 28. Chemistry. 2015. PMID: 26220197 Free PMC article.
References
-
- Godula K, Sames D. Science. 2006;312:67. - PubMed
-
-
Pastine SJ, Gribkov DV, Sames D. J Am Chem Soc. 2006;128:14220–14221.Gribkov DV, Pastine SJ, Schnurch M, Sames D. J Am Chem Soc. 2007;129:11750–11755.Review on transition metal-catalyzed α-functionalization of amines: Campos KR. Chem Soc Rev. 2007;36:1069–1084.
-
-
-
For examples of oxidative a-arylation of cyclic amines: Baslé O, Li C-J. Org Lett. 2008;10:3661–3663.and references therein.
-
-
-
During the preparation of this manuscript a report concerning the HT-amination of aromatic aldehydes was published in Organic Letters: Mori K, Kawasaki T, Akiyama T. Org Lett. 2012;14:1436–1439.
-
-
- Horton DA, Bourne GT, Smythe ML. Chem Rev. 2003;103:893–930. - PubMed
- Scott JD, Williams RM. Chem Rev. 2002;102:1669–1730. - PubMed
- Stevenson GI, Huscroft I, MacLeod AM, Swain CJ, Cascier MA, Chicci GC, Grahm MI, Harrison T, Kelleher FJ, Kurtz M, Ladduwahetty T, Merchant KJ, Metzger JM, Macintyre DE, Sadowski S, Sohal B, Owens AP. J Med Chem. 1998;41:4623–4635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

