C-H bond functionalization via hydride transfer: formation of α-arylated piperidines and 1,2,3,4-tetrahydroisoquinolines via stereoselective intramolecular amination of benzylic C-H bonds
- PMID: 22672002
- PMCID: PMC3433405
- DOI: 10.1021/jo300635m
C-H bond functionalization via hydride transfer: formation of α-arylated piperidines and 1,2,3,4-tetrahydroisoquinolines via stereoselective intramolecular amination of benzylic C-H bonds
Abstract
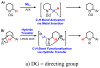
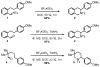
We here report a study of the intramolecular amination of sp(3) C-H bonds via the hydride transfer cyclization of N-tosylimines (HT-amination). In this transformation, 5-aryl aldehydes are subjected to N-toluenesulfonamide in the presence of BF(3)·OEt(2) to effect imine formation and HT-cyclization, leading to 2-arylpiperidines and 3-aryl-1,2,3,4-tetrahydroisoquinolines in a one-pot procedure. We examined the reactivity of a range of aldehyde substrates as a function of their conformational flexibility. Substrates of higher conformational rigidity were more reactive, giving higher yields of the desired products. However, a single substituent on the alkyl chain linking the N-tosylimine and the benzylic sp(3) C-H bonds was sufficient for HT-cyclization to occur. In addition, an examination of various arenes revealed that the electronic character of the hydridic C-H bonds dramatically affects the efficiency of the reaction. We also found that this transformation is highly stereoselective; 2-substituted aldehydes yield cis-2,5-disubstituted piperidines, while 3-substituted aldehydes afford trans-2,4-disubstituted piperidines. The stereoselectivity is a consequence of thermodynamic control. The pseudoallylic strain between the arene and tosyl group on the piperidine ring is proposed to rationalize the greater stability of the isomer with the aryl ring in the axial position. This preferential placement of the arene is proposed to affect the observed stereoselectivity.
Figures
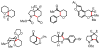
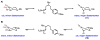
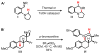


References
-
- Godula K, Sames D. Science. 2006;312:67. - PubMed
-
-
Pastine SJ, Gribkov DV, Sames D. J Am Chem Soc. 2006;128:14220–14221.Gribkov DV, Pastine SJ, Schnurch M, Sames D. J Am Chem Soc. 2007;129:11750–11755.Review on transition metal-catalyzed α-functionalization of amines: Campos KR. Chem Soc Rev. 2007;36:1069–1084.
-
-
-
For examples of oxidative a-arylation of cyclic amines: Baslé O, Li C-J. Org Lett. 2008;10:3661–3663.and references therein.
-
-
-
During the preparation of this manuscript a report concerning the HT-amination of aromatic aldehydes was published in Organic Letters: Mori K, Kawasaki T, Akiyama T. Org Lett. 2012;14:1436–1439.
-
-
- Horton DA, Bourne GT, Smythe ML. Chem Rev. 2003;103:893–930. - PubMed
- Scott JD, Williams RM. Chem Rev. 2002;102:1669–1730. - PubMed
- Stevenson GI, Huscroft I, MacLeod AM, Swain CJ, Cascier MA, Chicci GC, Grahm MI, Harrison T, Kelleher FJ, Kurtz M, Ladduwahetty T, Merchant KJ, Metzger JM, Macintyre DE, Sadowski S, Sohal B, Owens AP. J Med Chem. 1998;41:4623–4635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

