Salivary gland hypofunction induced by activation of innate immunity is dependent on type I interferon signaling
- PMID: 22672212
- PMCID: PMC3443546
- DOI: 10.1111/j.1600-0714.2012.01181.x
Salivary gland hypofunction induced by activation of innate immunity is dependent on type I interferon signaling
Abstract
Background: Activation of innate immunity through polyinosinic:polycytidylic acid [poly(I:C)] causes acute salivary gland hypofunction. As a major consequence of poly(I:C) treatment is type I interferon (IFN) production, this study was undertaken to investigate their role in salivary gland dysfunction.
Methods: Different strains of mice deficient in either interferon alpha receptor (IFNAR1(-/-)) or IL-6(-/-), or IL-10(-/-), or EBI3(-/-) were treated with poly(I:C). Salivary gland function was determined by measuring pilocarpine-induced saliva volume. Gene expression levels were measured by real-time PCR. Ca(2+) mobilization studies were performed using ex-vivo acinar cells.
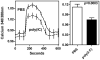
Results: A single injection of poly(I:C) rapidly induced salivary gland hypofunction in wild-type B6 mice (41% drop in saliva volumes compared to PBS-treated mice). In contrast, the loss of function in poly(I:C)-treated IFNAR(-/-) mice was only 9.6%. Gene expression analysis showed reduced levels of Il-6, Il-10, and Il-27 in submandibular glands of poly(I:C)-treated IFNAR(-/-) mice. While salivary gland dysfunction in poly(I:C)-treated IL-10(-/-) and EBI3(-/-) mice was comparable to wild-type mice, the IL-6(-/-) mice were more resistant, with only a 21% drop in function. Pilocarpine-induced Ca(2+) flux was significantly suppressed in acinar cells obtained from poly(I:C)-treated wild-type mice.
Conclusions: Our data demonstrate that a combined action of type I IFNs and IL-6 contributes toward salivary gland hypofunction. This happens through interference with Ca(2+) mobilization within acinar cells. Thus, in acute viral infections and diseases like Sjögren's syndrome, elevated levels of type I IFNs and IL-6 can directly affect glandular function.
© 2012 John Wiley & Sons A/S.
Conflict of interest statement
None of the authors have any conflict of interest.
Figures



Similar articles
-
Activation of innate immune responses through Toll-like receptor 3 causes a rapid loss of salivary gland function.J Oral Pathol Med. 2009 Jan;38(1):42-7. doi: 10.1111/j.1600-0714.2008.00700.x. J Oral Pathol Med. 2009. PMID: 19192049 Free PMC article.
-
Epithelial disruptions, but not immune cell invasion, induced secretory dysfunction following innate immune activation in a novel model of acute salivary gland injury.J Oral Pathol Med. 2018 Feb;47(2):211-219. doi: 10.1111/jop.12663. Epub 2017 Dec 18. J Oral Pathol Med. 2018. PMID: 29160910
-
Inducible nitric oxide synthase-mediated injury in a mouse model of acute salivary gland dysfunction.Nitric Oxide. 2018 Aug 1;78:95-102. doi: 10.1016/j.niox.2018.06.001. Epub 2018 Jun 7. Nitric Oxide. 2018. PMID: 29885902
-
Calcium signaling defects underlying salivary gland dysfunction.Biochim Biophys Acta Mol Cell Res. 2018 Nov;1865(11 Pt B):1771-1777. doi: 10.1016/j.bbamcr.2018.07.002. Epub 2018 Jul 10. Biochim Biophys Acta Mol Cell Res. 2018. PMID: 30006140 Review.
-
Salivary gland dysfunction and xerostomia in Sjögren's syndrome.Oral Maxillofac Surg Clin North Am. 2014 Feb;26(1):35-53. doi: 10.1016/j.coms.2013.09.003. Oral Maxillofac Surg Clin North Am. 2014. PMID: 24287192 Review.
Cited by
-
MICa/b-dependent activation of natural killer cells by CD64+ inflammatory type 2 dendritic cells contributes to autoimmunity.EMBO J. 2023 Dec 1;42(23):e113714. doi: 10.15252/embj.2023113714. Epub 2023 Nov 2. EMBO J. 2023. PMID: 37916875 Free PMC article.
-
Innate Immunity and Biological Therapies for the Treatment of Sjögren's Syndrome.Int J Mol Sci. 2020 Dec 1;21(23):9172. doi: 10.3390/ijms21239172. Int J Mol Sci. 2020. PMID: 33271951 Free PMC article. Review.
-
Pulmonary Involvement in a Mouse Model of Sjögren's Syndrome Induced by STING Activation.Int J Mol Sci. 2020 Jun 25;21(12):4512. doi: 10.3390/ijms21124512. Int J Mol Sci. 2020. PMID: 32630417 Free PMC article.
-
Biosemantics guided gene expression profiling of Sjögren's syndrome: a comparative analysis with systemic lupus erythematosus and rheumatoid arthritis.Arthritis Res Ther. 2017 Aug 17;19(1):192. doi: 10.1186/s13075-017-1400-3. Arthritis Res Ther. 2017. PMID: 28818099 Free PMC article.
-
Inhibition of NLRP3 inflammasome activity by MCC950 leads to exacerbation of Sjӧgren's syndrome pathologies in non-obese diabetic mice.Immunology. 2023 Apr;168(4):697-708. doi: 10.1111/imm.13605. Epub 2022 Nov 18. Immunology. 2023. PMID: 36353754 Free PMC article.
References
-
- HOPCRAFT MS, TAN C. Xerostomia: an update for clinicians. Aust Dent J. 2010;55:238–44. - PubMed
-
- PEDERSEN AM, REIBEL J, NAUNTOFTE B. Primary Sjogren's syndrome (pSS): subjective symptoms and salivary findings. J Oral Pathol Med. 1999;28:303–11. - PubMed
-
- BOOKMAN AA, SHEN H, COOK RJ, et al. Whole stimulated salivary flow: correlation with the pathology of inflammation and damage in minor salivary gland biopsy specimens from patients with primary Sjogren's syndrome but not patients with sicca. Arthritis Rheum. 2011;63:2014–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

