Significance of decoy receptor 3 (Dcr3) and external-signal regulated kinase 1/2 (Erk1/2) in gastric cancer
- PMID: 22672288
- PMCID: PMC3459731
- DOI: 10.1186/1471-2172-13-28
Significance of decoy receptor 3 (Dcr3) and external-signal regulated kinase 1/2 (Erk1/2) in gastric cancer
Abstract
Background: Decoy receptor 3 (DcR3), a member of the tumor necrosis factor receptor (TNFR) superfamily, is associated with anti-tumor immunity suppression. It is highly expressed in many tumors, and its expression can be regulated by the MAPK/MEK/ERK signaling pathway. The MAPK/MEK/ERK pathway has been reported to be a regulator in tumor occurrence, development and clonal expansion. External-signal regulated kinase (ERK) is a vital member of this pathway.
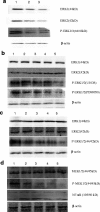
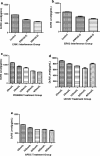
Results: The expression of DcR3 and ERK1/2 in tumor tissues of gastric cancer patients was significantly higher than the non-cancerous group (P < 0.05). There was no statistical difference among tumor tissues from patients with different ages or gender, and even of different differentiation (P > 0.05). However, in patients with stage I gastric cancer, the DcR3 and ERK1/2 levels were significantly lower than patients with more advanced stages.
Conclusions: DcR3 and ERK1/2 play a vital role in the development of gastric cancer, and they may be new markers for indicating the efficiency of gastric cancer treatment in the future.
Figures



Similar articles
-
Over-expression of decoy receptor 3 in gastric precancerous lesions and carcinoma.Ups J Med Sci. 2008;113(3):297-304. doi: 10.3109/2000-1967-240. Ups J Med Sci. 2008. PMID: 18991242
-
High DcR3 expression predicts stage pN2 in gastric cancer.Hepatogastroenterology. 2007 Oct-Nov;54(79):2172-6. Hepatogastroenterology. 2007. PMID: 18251184
-
High DcR3 expression predicts stage pN2-3 in gastric cancer.Am J Clin Oncol. 2008 Feb;31(1):79-83. doi: 10.1097/COC.0b013e3180ca77ad. Am J Clin Oncol. 2008. PMID: 18376232
-
Decoy receptor 3: an endogenous immunomodulator in cancer growth and inflammatory reactions.J Biomed Sci. 2017 Jun 19;24(1):39. doi: 10.1186/s12929-017-0347-7. J Biomed Sci. 2017. PMID: 28629361 Free PMC article. Review.
-
Decoy receptor 3: a pleiotropic immunomodulator and biomarker for inflammatory diseases, autoimmune diseases and cancer.Biochem Pharmacol. 2011 Apr 1;81(7):838-47. doi: 10.1016/j.bcp.2011.01.011. Epub 2011 Feb 2. Biochem Pharmacol. 2011. PMID: 21295012 Review.
Cited by
-
Overexpression of DcR3 and its significance on tumor cell differentiation and proliferation in glioma.ScientificWorldJournal. 2014 Mar 5;2014:605236. doi: 10.1155/2014/605236. eCollection 2014. ScientificWorldJournal. 2014. PMID: 24741354 Free PMC article.
-
The ERK1/2 signaling pathway is involved in sulfur dioxide preconditioning-induced protection against cardiac dysfunction in isolated perfused rat heart subjected to myocardial ischemia/reperfusion.Int J Mol Sci. 2013 Nov 8;14(11):22190-201. doi: 10.3390/ijms141122190. Int J Mol Sci. 2013. PMID: 24217229 Free PMC article.
-
Downregulation of DcR3 sensitizes hepatocellular carcinoma cells to TRAIL-induced apoptosis.Onco Targets Ther. 2017 Jan 18;10:417-428. doi: 10.2147/OTT.S127202. eCollection 2017. Onco Targets Ther. 2017. PMID: 28176915 Free PMC article.
-
Prognostic values of ERK1/2 and p-ERK1/2 expressions for poor survival in non-small cell lung cancer.Tumour Biol. 2015 Jun;36(6):4143-50. doi: 10.1007/s13277-015-3048-4. Epub 2015 Jan 18. Tumour Biol. 2015. PMID: 25596700
-
Weipixiao attenuate early angiogenesis in rats with gastric precancerous lesions.BMC Complement Altern Med. 2018 Sep 10;18(1):250. doi: 10.1186/s12906-018-2309-3. BMC Complement Altern Med. 2018. PMID: 30200948 Free PMC article.
References
-
- Roth W, Isenmann S, Nakamura M. et al.Soluble decoy receptor 3 is expressed by malignant gliomas and suppresses CD95 ligand-induced apoptosis and chemotaxis. Cancer Res. 2001;61:2759–2765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

