Tuning multiple motor travel via single motor velocity
- PMID: 22672518
- PMCID: PMC3418377
- DOI: 10.1111/j.1600-0854.2012.01385.x
Tuning multiple motor travel via single motor velocity
Abstract
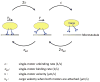
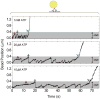
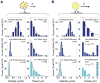
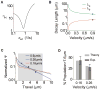
Microtubule-based molecular motors often work in small groups to transport cargos in cells. A key question in understanding transport (and its regulation in vivo) is to identify the sensitivity of multiple-motor-based motion to various single molecule properties. Whereas both single-motor travel distance and microtubule binding rate have been demonstrated to contribute to cargo travel, the role of single-motor velocity is yet to be explored. Here, we recast a previous theoretical study, and make explicit a potential contribution of velocity to cargo travel. We test this possibility experimentally, and demonstrate a strong negative correlation between single-motor velocity and cargo travel for transport driven by two motors. Our study thus discovers a previously unappreciated role of single-motor velocity in regulating multiple-motor transport.
© 2012 John Wiley & Sons A/S.
Figures
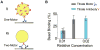
References
-
- Ashkin A, Schutze K, Dziedzic JM, Euteneuer U, Schliwa M. Force generation of organelle transport measured in vivo by an infrared laser trap. Nature. 1990;348:346–8. - PubMed
-
- Kural C, Kim H, Syed S, Goshima G, Gelfand VI, Selvin PR. Kinesin and dynein move a peroxisome in vivo: a tug-of-war or coordinated movement? Science. 2005;308:1469–72. - PubMed

