The HIV-1 Nef protein interacts with two components of the 40S small ribosomal subunit, the RPS10 protein and the 18S rRNA
- PMID: 22672539
- PMCID: PMC3393617
- DOI: 10.1186/1743-422X-9-103
The HIV-1 Nef protein interacts with two components of the 40S small ribosomal subunit, the RPS10 protein and the 18S rRNA
Abstract
Background: Human immunodeficiency virus type 1 (HIV-1) Nef-encoded protein plays key functions at almost all stages of the viral life cycle, but its role in translation is largely unknown.
Methods: To determine the effect of Nef on translation we used an in vitro translation assay. The detection of Nef/RPS10 complexes and the presence of 18S rRNA and tRNAs in the complexes were performed by coimmunoprecipitation and RT-PCR assay.
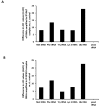
Results: We observed that the HIV-1 Nef protein specifically impaired translation in vitro. We observed the interaction of Nef with RPS10 by coimmunoprecipitation assay. In addition 18S rRNA and tRNAs were present in the Nef/RPS10 complexes.
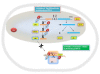
Conclusions: Our results are consistent with a model in which the Nef protein by binding to two components of the 40S small ribosomal subunit, RPS10 and 18S rRNA, and to a lesser extent to tRNAs, could lead to decreased protein synthesis.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

