Top 10 plant pathogenic bacteria in molecular plant pathology
- PMID: 22672649
- PMCID: PMC6638704
- DOI: 10.1111/j.1364-3703.2012.00804.x
Top 10 plant pathogenic bacteria in molecular plant pathology
Abstract
Many plant bacteriologists, if not all, feel that their particular microbe should appear in any list of the most important bacterial plant pathogens. However, to our knowledge, no such list exists. The aim of this review was to survey all bacterial pathologists with an association with the journal Molecular Plant Pathology and ask them to nominate the bacterial pathogens they would place in a 'Top 10' based on scientific/economic importance. The survey generated 458 votes from the international community, and allowed the construction of a Top 10 bacterial plant pathogen list. The list includes, in rank order: (1) Pseudomonas syringae pathovars; (2) Ralstonia solanacearum; (3) Agrobacterium tumefaciens; (4) Xanthomonas oryzae pv. oryzae; (5) Xanthomonas campestris pathovars; (6) Xanthomonas axonopodis pathovars; (7) Erwinia amylovora; (8) Xylella fastidiosa; (9) Dickeya (dadantii and solani); (10) Pectobacterium carotovorum (and Pectobacterium atrosepticum). Bacteria garnering honourable mentions for just missing out on the Top 10 include Clavibacter michiganensis (michiganensis and sepedonicus), Pseudomonas savastanoi and Candidatus Liberibacter asiaticus. This review article presents a short section on each bacterium in the Top 10 list and its importance, with the intention of initiating discussion and debate amongst the plant bacteriology community, as well as laying down a benchmark. It will be interesting to see, in future years, how perceptions change and which bacterial pathogens enter and leave the Top 10.
© 2012 The Authors. Molecular Plant Pathology © 2012 BSPP and Blackwell Publishing Ltd.
Figures








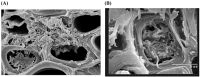
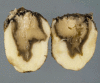



References
-
- Antunez‐Lamas, M. , Cabrera, E. , Lopez‐Solanilla, E. , Solano, R. , Gonzalez‐Melendi, P. , Chico, J.M. , Toth, I.K. , Birch, P.R.J. , Pritchard, L. , Liu, H. and Rodriguez‐Palenzuela, P. (2009) Bacterial chemoattraction towards jasmonate plays a role in the entry of Dickeya dadantii through wounded tissues. Mol. Microbiol. 74, 662–671. - PubMed
-
- Babujee, L. , Venkatesh, B. , Yamazaki, A. and Tsuyumu, S. (2007) Proteomic analysis of the carbonate insoluble outer membrane fraction of the soft‐rot pathogen Dickeya dadantii (syn. Erwinia chrysanthemi) strain 3937. J. Proteome Res. 6, 62–69. - PubMed
-
- Baltrus, D.A. , Nishimura, M.T. , Romanchuk, A. , Chang, J.H. , Mukhtar, M.S. , Cherkis, K. , Roach, J. , Grant, S.R. , Jones, C.D. and Dangl, J.L. (2011) Dynamic evolution of pathogenicity revealed by sequencing and comparative genomics of 19 Pseudomonas syringae isolates. PLoS Pathog. 7, e1002132. Epub: 1 July 2011. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

