EGF-like factors induce expansion of the cumulus cell-oocyte complexes by activating calpain-mediated cell movement
- PMID: 22673225
- PMCID: PMC3404342
- DOI: 10.1210/en.2012-1059
EGF-like factors induce expansion of the cumulus cell-oocyte complexes by activating calpain-mediated cell movement
Abstract
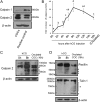
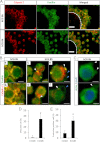
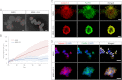
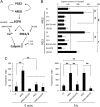
Cumulus cell-oocyte complex (COC) expansion is obligatory for LH-induced ovulation and is initiated by LH induction of the epidermal growth factor (EGF)-like factors that mediate the synthesis of the hyaluronan-rich matrix and hyaluronan-stabilizing factors. COC expansion also involves the movement of cumulus cells within the matrix by mechanisms that have not been characterized. We document herein that two proteases, calpain 2 and to a lesser extent calpain 1, are expressed in cumulus cells and that the proteolytic activity of these enzymes is rapidly and significantly increased in COC isolated from human chorionic gonadotropin-induced ovulatory follicles in vivo. Stimulation of calpain activity was associated with proteolytic degradation of paxillin and talin (two components of focal adhesion complexes), cell detachment, and the formation of cell surface bleb-like protrusions. Injection of a calpain inhibitor in vivo reduced 1) human chorionic gonadotropin-stimulated calpain enzyme activity, 2) cell detachment, 3) membrane protrusion formation, and 4) COC expansion by mechanisms that did not alter Has2 expression. During EGF-like factor induction of COC expansion in culture, calpain activity was increased by ERK1/2 and intracellular Ca(2+) signaling pathways. Inhibition of calpain activity in cultured COC blocked cumulus cell detachment, protrusion formation, and the vigorous movement of cumulus cells. As a consequence, COC expansion was impaired. Collectively, these results show that two highly coordinated processes control COC expansion. One process involves the synthesis of the hyaluronan matrix, and the other mediates cumulus cell detachment and movement. The latter are controlled by calpain activation downstream of the EGF receptor activation of the Ca(2+) pathway and ERK1/2 pathways.
Figures

Similar articles
-
Knockdown of TrkA in cumulus oocyte complexes (COCs) inhibits EGF-induced cumulus expansion by down-regulation of IL-6.Mol Cell Endocrinol. 2014 Feb 15;382(2):804-13. doi: 10.1016/j.mce.2013.10.031. Epub 2013 Nov 8. Mol Cell Endocrinol. 2014. PMID: 24215827
-
Hormone-induced expression of tumor necrosis factor alpha-converting enzyme/A disintegrin and metalloprotease-17 impacts porcine cumulus cell oocyte complex expansion and meiotic maturation via ligand activation of the epidermal growth factor receptor.Endocrinology. 2007 Dec;148(12):6164-75. doi: 10.1210/en.2007-0195. Epub 2007 Sep 27. Endocrinology. 2007. PMID: 17901238
-
Epiregulin can effectively mature isolated cumulus-oocyte complexes, but fails as a substitute for the hCG/epidermal growth factor stimulus on cultured follicles.Reproduction. 2009 Jun;137(6):997-1005. doi: 10.1530/REP-08-0523. Epub 2009 Mar 25. Reproduction. 2009. PMID: 19321658
-
Regulation of cumulus expansion and hyaluronan synthesis in porcine oocyte-cumulus complexes during in vitro maturation.Endocr Regul. 2012 Oct;46(4):225-35. doi: 10.4149/endo_2012_04_225. Endocr Regul. 2012. PMID: 23127506 Review.
-
The release of EGF domain from EGF-like factors by a specific cleavage enzyme activates the EGFR-MAPK3/1 pathway in both granulosa cells and cumulus cells during the ovulation process.J Reprod Dev. 2012;58(5):510-4. doi: 10.1262/jrd.2012-056. J Reprod Dev. 2012. PMID: 23124701 Review.
Cited by
-
Embryonic poly(A)-binding protein interacts with translation-related proteins and undergoes phosphorylation on the serine, threonine, and tyrosine residues in the mouse oocytes and early embryos.J Assist Reprod Genet. 2023 Apr;40(4):929-941. doi: 10.1007/s10815-023-02746-7. Epub 2023 Feb 24. J Assist Reprod Genet. 2023. PMID: 36823316 Free PMC article.
-
FSH in vitro versus LH in vivo: similar genomic effects on the cumulus.J Ovarian Res. 2013 Sep 25;6(1):68. doi: 10.1186/1757-2215-6-68. J Ovarian Res. 2013. PMID: 24066945 Free PMC article.
-
Bovine In Vitro Oocyte Maturation and Embryo Production Used as a Model for Testing Endocrine Disrupting Chemicals Eliciting Female Reproductive Toxicity With Diethylstilbestrol as a Showcase Compound.Front Toxicol. 2022 May 24;4:811285. doi: 10.3389/ftox.2022.811285. eCollection 2022. Front Toxicol. 2022. PMID: 35686045 Free PMC article.
-
Extracellular Vesicles from Bovine Follicular Fluid Support Cumulus Expansion.Biol Reprod. 2015 Nov;93(5):117. doi: 10.1095/biolreprod.115.132977. Epub 2015 Sep 30. Biol Reprod. 2015. PMID: 26423123 Free PMC article.
-
The molecular regulatory mechanisms of meiotic arrest and resumption in Oocyte development and maturation.Reprod Biol Endocrinol. 2023 Oct 2;21(1):90. doi: 10.1186/s12958-023-01143-0. Reprod Biol Endocrinol. 2023. PMID: 37784186 Free PMC article. Review.
References
-
- Eppig JJ. 1991. Inter communication between mammalian oocytes and companion somatic cells. Bioessays 13:569–574 - PubMed
-
- Kidder GM, Mhawi AA. 2002. Gap junctions and ovarian folliculogenesis. Reproduction 123:613–620 - PubMed
-
- Larsen WJ, Wert SE, Brunner GD. 1987. Differential modulation of rat follicle cell gap junction populations at ovulation. Dev Biol 122:61–71 - PubMed
-
- Dekel N, Kraicer PF. 1978. Induction in vitro of mucification of rat cumulus oophorus by gonadotrophins and adenosine 3′,5′-monophosphate. Endocrinology 102:1797–1802 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

