Sphingosine 1-phosphate receptor 2 antagonist JTE-013 increases the excitability of sensory neurons independently of the receptor
- PMID: 22673325
- PMCID: PMC3544961
- DOI: 10.1152/jn.00825.2011
Sphingosine 1-phosphate receptor 2 antagonist JTE-013 increases the excitability of sensory neurons independently of the receptor
Abstract
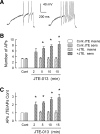
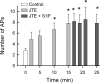
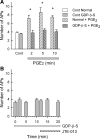
Previously we demonstrated that sphingosine 1-phosphate receptor 1 (S1PR(1)) played a prominent, but not exclusive, role in enhancing the excitability of small-diameter sensory neurons, suggesting that other S1PRs can modulate neuronal excitability. To examine the potential role of S1PR(2) in regulating neuronal excitability we used the established selective antagonist of S1PR(2), JTE-013. Here we report that exposure to JTE-013 alone produced a significant increase in excitability in a time- and concentration-dependent manner in 70-80% of recorded neurons. Internal perfusion of sensory neurons with guanosine 5'-O-(2-thiodiphosphate) (GDP-β-S) via the recording pipette inhibited the sensitization produced by JTE-013 as well as prostaglandin E(2). Pretreatment with pertussis toxin or the selective S1PR(1) antagonist W146 blocked the sensitization produced by JTE-013. These results indicate that JTE-013 might act as an agonist at other G protein-coupled receptors. In neurons that were sensitized by JTE-013, single-cell RT-PCR studies demonstrated that these neurons did not express the mRNA for S1PR(2). In behavioral studies, injection of JTE-013 into the rat's hindpaw produced a significant increase in the mechanical sensitivity in the ipsilateral, but not contralateral, paw. Injection of JTE-013 did not affect the withdrawal latency to thermal stimulation. Thus JTE-013 augments neuronal excitability independently of S1PR(2) by unknown mechanisms that may involve activation of other G protein-coupled receptors such as S1PR(1). Clearly, further studies are warranted to establish the causal nature of this increased sensitivity, and future studies of neuronal function using JTE-013 should be interpreted with caution.
Figures





Similar articles
-
Sphingosine 1-phosphate enhances the excitability of rat sensory neurons through activation of sphingosine 1-phosphate receptors 1 and/or 3.J Neuroinflammation. 2015 Apr 12;12:70. doi: 10.1186/s12974-015-0286-8. J Neuroinflammation. 2015. PMID: 25880547 Free PMC article.
-
The sphingosine 1-phosphate receptor, S1PR₁, plays a prominent but not exclusive role in enhancing the excitability of sensory neurons.J Neurophysiol. 2010 Nov;104(5):2741-8. doi: 10.1152/jn.00709.2010. Epub 2010 Sep 15. J Neurophysiol. 2010. PMID: 20844107 Free PMC article.
-
Role for peroxynitrite in sphingosine-1-phosphate-induced hyperalgesia in rats.Pain. 2011 Mar;152(3):643-648. doi: 10.1016/j.pain.2010.12.011. Epub 2011 Jan 15. Pain. 2011. PMID: 21239112 Free PMC article.
-
Sphingosine-1-phosphate via activation of a G-protein-coupled receptor(s) enhances the excitability of rat sensory neurons.J Neurophysiol. 2006 Sep;96(3):1042-52. doi: 10.1152/jn.00120.2006. Epub 2006 May 24. J Neurophysiol. 2006. PMID: 16723416
-
Expression of sphingosine 1-phosphate receptors in the rat dorsal root ganglia and defined single isolated sensory neurons.Physiol Genomics. 2012 Sep 18;44(18):889-901. doi: 10.1152/physiolgenomics.00053.2012. Epub 2012 Jul 17. Physiol Genomics. 2012. PMID: 22805346 Free PMC article.
Cited by
-
Sphingosine-1-Phosphate Receptor-2 Antagonists: Therapeutic Potential and Potential Risks.Front Pharmacol. 2016 Jun 21;7:167. doi: 10.3389/fphar.2016.00167. eCollection 2016. Front Pharmacol. 2016. PMID: 27445808 Free PMC article. Review.
-
Chemical and genetic tools to explore S1P biology.Curr Top Microbiol Immunol. 2014;378:55-83. doi: 10.1007/978-3-319-05879-5_3. Curr Top Microbiol Immunol. 2014. PMID: 24728593 Free PMC article. Review.
-
A potent and selective C-11 labeled PET tracer for imaging sphingosine-1-phosphate receptor 2 in the CNS demonstrates sexually dimorphic expression.Org Biomol Chem. 2015 Aug 7;13(29):7928-39. doi: 10.1039/c5ob00951k. Epub 2015 Jun 25. Org Biomol Chem. 2015. PMID: 26108234 Free PMC article.
-
The Repertoire of Small-Molecule PET Probes for Neuroinflammation Imaging: Challenges and Opportunities beyond TSPO.J Med Chem. 2021 Dec 23;64(24):17656-17689. doi: 10.1021/acs.jmedchem.1c01571. Epub 2021 Dec 14. J Med Chem. 2021. PMID: 34905377 Free PMC article. Review.
-
Sphingosine 1-phosphate enhances the excitability of rat sensory neurons through activation of sphingosine 1-phosphate receptors 1 and/or 3.J Neuroinflammation. 2015 Apr 12;12:70. doi: 10.1186/s12974-015-0286-8. J Neuroinflammation. 2015. PMID: 25880547 Free PMC article.
References
-
- Alderton F, Rakhit S, Kong KC, Palmer T, Sambi B, Pyne S, Pyne NJ. Tethering of the platelet-derived growth factor beta receptor to G-protein-coupled receptors. A novel platform for integrative signaling by these receptor classes in mammalian cells. J Biol Chem 276: 28578–28585, 2001 - PubMed
-
- Anliker B, Chun J. Cell surface receptors in lysophospholipid signaling. Semin Cell Dev Biol 15: 457–465, 2004 - PubMed
-
- Arikawa K, Takuwa N, Yamaguchi H, Sugimoto N, Kitayama J, Nagawa H, Takehara K, Takuwa Y. Ligand-dependent inhibition of B16 melanoma cell migration and invasion via endogenous S1P2 G protein-coupled receptor. Requirement of inhibition of cellular RAC activity. J Biol Chem 278: 32841–32851, 2003 - PubMed
-
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 277: 21453–21457, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

