Force encoding in stick insect legs delineates a reference frame for motor control
- PMID: 22673329
- PMCID: PMC3774582
- DOI: 10.1152/jn.00274.2012
Force encoding in stick insect legs delineates a reference frame for motor control
Abstract
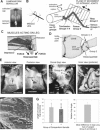
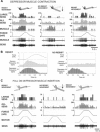
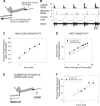
The regulation of forces is integral to motor control. However, it is unclear how information from sense organs that detect forces at individual muscles or joints is incorporated into a frame of reference for motor control. Campaniform sensilla are receptors that monitor forces by cuticular strains. We studied how loads and muscle forces are encoded by trochanteral campaniform sensilla in stick insects. Forces were applied to the middle leg to emulate loading and/or muscle contractions. Selective sensory ablations limited activities recorded in the main leg nerve to specific receptor groups. The trochanteral campaniform sensilla consist of four discrete groups. We found that the dorsal groups (Groups 3 and 4) encoded force increases and decreases in the plane of movement of the coxo-trochanteral joint. Group 3 receptors discharged to increases in dorsal loading and decreases in ventral load. Group 4 showed the reverse directional sensitivities. Vigorous, directional responses also occurred to contractions of the trochanteral depressor muscle and to forces applied at the muscle insertion. All sensory discharges encoded the amplitude and rate of loading or muscle force. Stimulation of the receptors produced reflex effects in the depressor motoneurons that could reverse in sign during active movements. These data, in conjunction with findings of previous studies, support a model in which the trochanteral receptors function as an array that can detect forces in all directions relative to the intrinsic plane of leg movement. The array could provide requisite information about forces and simplify the control and adaptation of posture and walking.
Figures






References
-
- Akay T. The Role of Sensory Signals for Interjoint Coordination in Stick Insect Legs (Carausius morosus and Cuniculina impigra) (PhD thesis). Cologne, Germany: University of Cologne, 2002
-
- Akay T, Bässler U, Gerharz P, Büschges A. The role of sensory signals from the insect coxa-trochanteral joint in controlling motor activity of the femur-tibia joint. J Neurophysiol 85: 594–604, 2001 - PubMed
-
- Akay T, Büschges A. Load signals assist the generation of movement-dependent reflex reversal in the femur-tibia joint of stick insects. J Neurophysiol 96: 3532–3537, 2006 - PubMed
-
- Akay T, Haehn S, Schmitz J, Büschges A. Signals from load sensors underlie interjoint coordination during stepping movements of the stick insect leg. J Neurophysiol 92: 42–51, 2004 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

