Inhibition of Src kinase activity attenuates amyloid associated microgliosis in a murine model of Alzheimer's disease
- PMID: 22673542
- PMCID: PMC3388011
- DOI: 10.1186/1742-2094-9-117
Inhibition of Src kinase activity attenuates amyloid associated microgliosis in a murine model of Alzheimer's disease
Abstract
Background: Microglial activation is an important histologic characteristic of the pathology of Alzheimer's disease (AD). One hypothesis is that amyloid beta (Aβ) peptide serves as a specific stimulus for tyrosine kinase-based microglial activation leading to pro-inflammatory changes that contribute to disease. Therefore, inhibiting Aβ stimulation of microglia may prove to be an important therapeutic strategy for AD.
Methods: Primary murine microglia cultures and the murine microglia cell line, BV2, were used for stimulation with fibrillar Aβ1-42. The non-receptor tyrosine kinase inhibitor, dasatinib, was used to treat the cells to determine whether Src family kinase activity was required for the Aβ stimulated signaling response and subsequent increase in TNFα secretion using Western blot analysis and enzyme-linked immunosorbent assay (ELISA), respectively. A histologic longitudinal analysis was performed using an AD transgenic mouse model, APP/PS1, to determine an age at which microglial protein tyrosine kinase levels increased in order to administer dasatinib via mini osmotic pump diffusion. Effects of dasatinib administration on microglial and astroglial activation, protein phosphotyrosine levels, active Src kinase levels, Aβ plaque deposition, and spatial working memory were assessed via immunohistochemistry, Western blot, and T maze analysis.
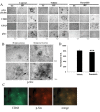
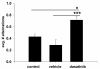
Results: Aβ fibrils stimulated primary murine microglia via a tyrosine kinase pathway involving Src kinase that was attenuated by dasatinib. Dasatinib administration to APP/PS1 mice decreased protein phosphotyrosine, active Src, reactive microglia, and TNFα levels in the hippocampus and temporal cortex. The drug had no effect on GFAP levels, Aβ plaque load, or the related tyrosine kinase, Lyn. These anti-inflammatory changes correlated with improved performance on the T maze test in dasatinib infused animals compared to control animals.
Conclusions: These data suggest that amyloid dependent microgliosis may be Src kinase dependent in vitro and in vivo. This study defines a role for Src kinase in the microgliosis characteristic of diseased brains and suggests that particular tyrosine kinase inhibition may be a valid anti-inflammatory approach to disease. Dasatinib is an FDA-approved drug for treating chronic myeloid leukemia cancer with a reported ability to cross the blood-brain barrier. Therefore, this suggests a novel use for this drug as well as similar acting molecules.
Figures








Similar articles
-
Amyloid-β oligomers stimulate microglia through a tyrosine kinase dependent mechanism.Neurobiol Aging. 2012 Oct;33(10):2247-61. doi: 10.1016/j.neurobiolaging.2011.10.027. Epub 2011 Dec 1. Neurobiol Aging. 2012. PMID: 22133278 Free PMC article.
-
Characterization of Novel Src Family Kinase Inhibitors to Attenuate Microgliosis.PLoS One. 2015 Jul 10;10(7):e0132604. doi: 10.1371/journal.pone.0132604. eCollection 2015. PLoS One. 2015. PMID: 26161952 Free PMC article.
-
Attenuation of microglial activation in a mouse model of Alzheimer's disease via NFAT inhibition.J Neuroinflammation. 2015 Mar 4;12:42. doi: 10.1186/s12974-015-0255-2. J Neuroinflammation. 2015. PMID: 25889879 Free PMC article.
-
NADPH oxidase as a therapeutic target in Alzheimer's disease.BMC Neurosci. 2008 Dec 3;9 Suppl 2(Suppl 2):S8. doi: 10.1186/1471-2202-9-S2-S8. BMC Neurosci. 2008. PMID: 19090996 Free PMC article. Review.
-
Targeting SRC in glioblastoma tumors and brain metastases: rationale and preclinical studies.Cancer Lett. 2010 Dec 8;298(2):139-49. doi: 10.1016/j.canlet.2010.08.014. Cancer Lett. 2010. PMID: 20947248 Free PMC article. Review.
Cited by
-
The VEGF inhibitor vatalanib regulates AD pathology in 5xFAD mice.Mol Brain. 2020 Sep 25;13(1):131. doi: 10.1186/s13041-020-00673-7. Mol Brain. 2020. PMID: 32977842 Free PMC article.
-
PDB_Amyloid: an extended live amyloid structure list from the PDB.FEBS Open Bio. 2018 Nov 22;9(1):185-190. doi: 10.1002/2211-5463.12524. eCollection 2019 Jan. FEBS Open Bio. 2018. PMID: 30652085 Free PMC article.
-
Manganese exposure exacerbates progressive motor deficits and neurodegeneration in the MitoPark mouse model of Parkinson's disease: Relevance to gene and environment interactions in metal neurotoxicity.Neurotoxicology. 2018 Jan;64:240-255. doi: 10.1016/j.neuro.2017.06.002. Epub 2017 Jun 20. Neurotoxicology. 2018. PMID: 28595911 Free PMC article.
-
The Dual Role of Microglia in Blood-Brain Barrier Dysfunction after Stroke.Curr Neuropharmacol. 2020;18(12):1237-1249. doi: 10.2174/1570159X18666200529150907. Curr Neuropharmacol. 2020. PMID: 32469699 Free PMC article. Review.
-
Overexpression of mutant amyloid-β protein precursor and presenilin 1 modulates enteric nervous system.J Alzheimers Dis. 2015;44(4):1263-78. doi: 10.3233/JAD-142259. J Alzheimers Dis. 2015. PMID: 25408221 Free PMC article.
References
-
- Akiyama H, Mori H, Saido T, Kondo H, Ikeda K, McGeer PL. Occurrence of the diffuse amyloid beta-protein (Abeta) deposits with numerous Abeta-containing glial cells in the cerebral cortex of patients with Alzheimer's disease. Glia. 1999;25:324–331. doi: 10.1002/(SICI)1098-1136(19990215)25:4<324::AID-GLIA2>3.0.CO;2-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

