Inflammation alters regional mitochondrial Ca²+ in human airway smooth muscle cells
- PMID: 22673614
- PMCID: PMC3423021
- DOI: 10.1152/ajpcell.00414.2011
Inflammation alters regional mitochondrial Ca²+ in human airway smooth muscle cells
Abstract
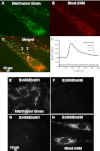
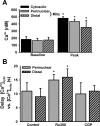
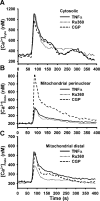
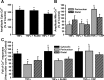
Regulation of cytosolic Ca(2+) concentration ([Ca(2+)](cyt)) in airway smooth muscle (ASM) is a key aspect of airway contractility and can be modulated by inflammation. Mitochondria have tremendous potential for buffering [Ca(2+)](cyt), helping prevent Ca(2+) overload, and modulating other intracellular events. Here, compartmentalization of mitochondria to different cellular regions may subserve different roles. In the present study, we examined the role of Ca(2+) buffering by mitochondria and mitochondrial Ca(2+) transport mechanisms in the regulation of [Ca(2+)](cyt) in enzymatically dissociated human ASM cells upon exposure to the proinflammatory cytokines TNF-α and IL-13. Cells were loaded simultaneously with fluo-3 AM and rhod-2 AM, and [Ca(2+)](cyt) and mitochondrial Ca(2+) concentration ([Ca(2+)](mito)) were measured, respectively, using real-time two-color fluorescence microscopy in both the perinuclear and distal, perimembranous regions of cells. Histamine induced a rapid increase in both [Ca(2+)](cyt) and [Ca(2+)](mito), with a significant delay in the mitochondrial response. Inhibition of the mitochondrial Na(+)/Ca(2+) exchanger (1 μM CGP-37157) increased [Ca(2+)](mito) responses in perinuclear mitochondria but not distal mitochondria. Inhibition of the mitochondrial uniporter (1 μM Ru360) decreased [Ca(2+)](mito) responses in perinuclear and distal mitochondria. CGP-37157 and Ru360 significantly enhanced histamine-induced [Ca(2+)](cyt). TNF-α and IL-13 both increased [Ca(2+)](cyt), which was associated with decreased [Ca(2+)](mito) in the case of TNF-α but not IL-13. The effects of TNF-α on both [Ca(2+)](cyt) and [Ca(2+)](mito) were affected by CGP-37157 but not by Ru360. Overall, these data demonstrate that in human ASM cells, mitochondria buffer [Ca(2+)](cyt) after agonist stimulation and its enhancement by inflammation. The differential regulation of [Ca(2+)](mito) in different parts of ASM cells may serve to locally regulate Ca(2+) fluxes from intracellular sources versus the plasma membrane as well as respond to differential energy demands at these sites. We propose that such differential mitochondrial regulation, and its disruption, may play a role in airway hyperreactivity in diseases such as asthma, where [Ca(2+)](cyt) is increased.
Figures





Similar articles
-
TNFα decreases mitochondrial movement in human airway smooth muscle.Am J Physiol Lung Cell Mol Physiol. 2017 Jul 1;313(1):L166-L176. doi: 10.1152/ajplung.00538.2016. Epub 2017 May 4. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28473328 Free PMC article.
-
Caveolin-1 in cytokine-induced enhancement of intracellular Ca(2+) in human airway smooth muscle.Am J Physiol Lung Cell Mol Physiol. 2011 Oct;301(4):L607-14. doi: 10.1152/ajplung.00019.2011. Epub 2011 Jul 29. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21803870 Free PMC article.
-
Mitochondrial modulation of Ca2+ sparks and transient KCa currents in smooth muscle cells of rat cerebral arteries.J Physiol. 2004 May 1;556(Pt 3):755-71. doi: 10.1113/jphysiol.2003.059568. Epub 2004 Feb 6. J Physiol. 2004. PMID: 14766935 Free PMC article.
-
Interaction between endoplasmic/sarcoplasmic reticulum stress (ER/SR stress), mitochondrial signaling and Ca(2+) regulation in airway smooth muscle (ASM).Can J Physiol Pharmacol. 2015 Feb;93(2):97-110. doi: 10.1139/cjpp-2014-0361. Epub 2014 Nov 25. Can J Physiol Pharmacol. 2015. PMID: 25506723 Free PMC article. Review.
-
Cross Talk Between Mitochondrial Reactive Oxygen Species and Sarcoplasmic Reticulum Calcium in Pulmonary Arterial Smooth Muscle Cells.Adv Exp Med Biol. 2017;967:289-298. doi: 10.1007/978-3-319-63245-2_17. Adv Exp Med Biol. 2017. PMID: 29047093 Review.
Cited by
-
Reactive Oxygen Species Behaving Badly: Oxidized Phosphatidylcholines Corrupt Ca2+ Signaling in Airway Smooth Muscle.Am J Respir Cell Mol Biol. 2023 Dec;69(6):605-607. doi: 10.1165/rcmb.2023-0295ED. Am J Respir Cell Mol Biol. 2023. PMID: 37672671 Free PMC article. No abstract available.
-
TNFα decreases mitochondrial movement in human airway smooth muscle.Am J Physiol Lung Cell Mol Physiol. 2017 Jul 1;313(1):L166-L176. doi: 10.1152/ajplung.00538.2016. Epub 2017 May 4. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28473328 Free PMC article.
-
Update on Calcium Signaling in Cystic Fibrosis Lung Disease.Front Pharmacol. 2021 Mar 11;12:581645. doi: 10.3389/fphar.2021.581645. eCollection 2021. Front Pharmacol. 2021. PMID: 33776759 Free PMC article. Review.
-
Mitochondrial Calcium Uniporter Structure and Function in Different Types of Muscle Tissues in Health and Disease.Int J Mol Sci. 2019 Sep 28;20(19):4823. doi: 10.3390/ijms20194823. Int J Mol Sci. 2019. PMID: 31569359 Free PMC article. Review.
-
Cell-Based Measurement of Mitochondrial Function in Human Airway Smooth Muscle Cells.Int J Mol Sci. 2023 Jul 15;24(14):11506. doi: 10.3390/ijms241411506. Int J Mol Sci. 2023. PMID: 37511264 Free PMC article.
References
-
- Amrani Y. TNF-alpha and calcium signaling in airway smooth muscle cells: a never-ending story with promising therapeutic relevance. Am J Respir Cell Mol Biol 36: 387–388, 2007 - PubMed
-
- Amrani Y, Panettieri RA., Jr Modulation of calcium homeostasis as a mechanism for altering smooth muscle responsiveness in asthma. Curr Opin Allergy Clin Immunol 2: 39–45, 2002 - PubMed
-
- Ay B, Prakash YS, Pabelick CM, Sieck GC. Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L909–L917, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

