Melanoma induction by ultraviolet A but not ultraviolet B radiation requires melanin pigment
- PMID: 22673911
- PMCID: PMC3621412
- DOI: 10.1038/ncomms1893
Melanoma induction by ultraviolet A but not ultraviolet B radiation requires melanin pigment
Abstract
Malignant melanoma of the skin (CMM) is associated with ultraviolet radiation exposure, but the mechanisms and even the wavelengths responsible are unclear. Here we use a mammalian model to investigate melanoma formed in response to precise spectrally defined ultraviolet wavelengths and biologically relevant doses. We show that melanoma induction by ultraviolet A (320-400 nm) requires the presence of melanin pigment and is associated with oxidative DNA damage within melanocytes. In contrast, ultraviolet B radiation (280-320 nm) initiates melanoma in a pigment-independent manner associated with direct ultraviolet B DNA damage. Thus, we identified two ultraviolet wavelength-dependent pathways for the induction of CMM and describe an unexpected and significant role for melanin within the melanocyte in melanomagenesis.
Figures

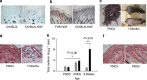

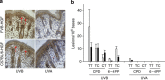
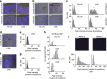
Comment in
-
Melanoma back in the UVA spotlight.Pigment Cell Melanoma Res. 2012 Sep;25(5):540-1. doi: 10.1111/j.1755-148X.2012.01028.x. Pigment Cell Melanoma Res. 2012. PMID: 22862995 No abstract available.
References
-
- Whiteman D. C., Whiteman C. A. & Green A. C. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies. Cancer Causes Control 12, 69–82 (2001). - PubMed
-
- Hocker T. & Tsao H. Ultraviolet radiation and melanoma: a systematic review and analysis of reported sequence variants. Hum. Mutat. 28, 578–588 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

