Cystogenesis and elongated primary cilia in Tsc1-deficient distal convoluted tubules
- PMID: 22674026
- PMCID: PMC3423116
- DOI: 10.1152/ajprenal.00141.2012
Cystogenesis and elongated primary cilia in Tsc1-deficient distal convoluted tubules
Abstract
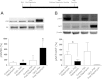
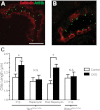
Tuberous sclerosis complex (TSC) is a multiorgan hamartomatous disease caused by loss of function mutations of either the TSC1 or TSC2 genes. Neurological symptoms of TSC predominate in younger patients, but renal pathologies are a serious aspect of the disease in older children and adults. To study TSC pathogenesis in the kidney, we inactivated the mouse Tsc1 gene in the distal convoluted tubules (DCT). At young ages, Tsc1 conditional knockout (CKO) mice have enlarged kidneys and mild cystogenesis with increased mammalian target of rapamycin complex (mTORC)1 but decreased mTORC2 signaling. Treatment with the mTORC1 inhibitor rapamycin reduces kidney size and cystogenesis. Rapamycin withdrawal led to massive cystogenesis involving both distal as well as proximal tubules. To assess the contribution of decreased mTORC2 signaling in kidney pathogenesis, we also generated Rictor CKO mice. These animals did not have any detectable kidney pathology. Finally, we examined primary cilia in the DCT. Cilia were longer in Tsc1 CKO mice, and rapamycin treatment returned cilia length to normal. Rictor CKO mice had normal cilia in the DCT. Overall, our findings suggest that loss of the Tsc1 gene in the DCT is sufficient for renal cystogenesis. This cytogenesis appears to be mTORC1 but not mTORC2 dependent. Intriguingly, the mechanism may be cell autonomous as well as non-cell autonomous and possibly involves the length and function of primary cilia.
Figures




References
-
- Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet 7: 125– 148, 2006 - PubMed
-
- Briata P, Di Blas E, Gulisano M, Mallamaci A, Iannone R, Boncinelli E, Corte G. EMX1 homeoprotein is expressed in cell nuclei of the developing cerebral cortex and in the axons of the olfactory sensory neurons. Mech Dev 57: 169– 180, 1996 - PubMed
-
- Consortium of European Chromosome 16 Tuberous Sclerosis Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 75: 1305– 1315, 1993 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

