Adherens junctions in C. elegans embryonic morphogenesis
- PMID: 22674076
- PMCID: PMC3633470
- DOI: 10.1007/978-94-007-4186-7_12
Adherens junctions in C. elegans embryonic morphogenesis
Abstract
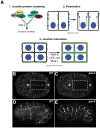
Caenorhabditis elegans provides a simplified, in vivo model system in which to study adherens junctions (AJs) and their role in morphogenesis. The core AJ components-HMR-1/E-cadherin, HMP-2/β-catenin and HMP-1/α-catenin-were initially identified through genetic screens for mutants with body axis elongation defects. In early embryos, AJ proteins are found at sites of contact between blastomeres, and in epithelial cells AJ proteins localize to the multifaceted apical junction (CeAJ)-a single structure that combines the adhesive and barrier functions of vertebrate adherens and tight junctions. The apically localized polarity proteins PAR-3 and PAR-6 mediate formation and maturation of junctions, while the basolaterally localized regulator LET-413/Scribble ensures that junctions remain apically positioned. AJs promote robust adhesion between epithelial cells and provide mechanical resistance for the physical strains of morphogenesis. However, in contrast to vertebrates, C. elegans AJ proteins are not essential for general cell adhesion or for epithelial cell polarization. A combination of conserved and novel proteins localizes to the CeAJ and works together with AJ proteins to mediate adhesion.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

