Lesion kinetics in a non-human primate model of endometriosis
- PMID: 22674203
- PMCID: PMC3398680
- DOI: 10.1093/humrep/des196
Lesion kinetics in a non-human primate model of endometriosis
Abstract
Background: Endometriosis is a common cause of pelvic pain and infertility in women of reproductive age. It is characterized by the presence of endometrial tissue outside the normal location, predominantly in the pelvic peritoneum causing severe abdominal pain. However, the severity of the symptoms of endometriosis does not always correlate with the anatomic severity of the disease. This lack of correlation may be due to morphological lesion variation during disease progression. This study examined lesion kinetics in a non-human primate model of endometriosis to better understand lesion dynamics.
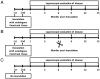
Methods: Endometriosis was experimentally induced in nine normal cycling female adult olive baboons (Papio anubis) by i.p. inoculation of autologous menstrual endometrium on Day 2 of menses for two consecutive menstrual cycles. Diagnostic laparoscopies were performed between Day 8-12 post-ovulation at 1, 3, 6, 9 and 12 months, followed by a necropsy at 15 months, after the second inoculation. In two animals, lesions were excised/ablated at 6 months and they were monitored for lesion recurrence and morphological changes by serial laparoscopy. Furthermore, five control animals underwent surgeries conducted at the same time points but without inoculation.
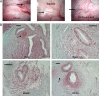
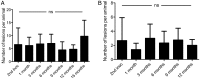
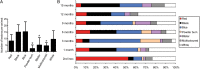
Results: A total of 542 endometriotic lesions were observed. The location, macroscopic (different colours) and microscopic appearance confirmed distinct endometriosis pathology in line with human disease. The majority of the lesions found 1 month after tissue inoculation were red lesions, which frequently changed colour during the disease progression. In contrast, blue lesions remained consistently blue while white lesions were evident at the later stages of the disease process and often regressed. There were significantly lower numbers of powder burn, blister and multicoloured lesions observed per animal in comparison to black and blue lesions (P-value≤0.05). New lesions were continually arising and persisted up to 15 months post-inoculation. Lesions reoccurred as early as 3 months after removal and 69% of lesions excised/ablated had reoccurred 9 months later. Interestingly, endometriotic lesions were also found in the non-inoculated animals, starting at the 6-month time point following multiple surgeries.
Conclusions: Documentation of lesion turnover in baboons indicated that lesions changed their colour from red to white over time. Different lesion types underwent metamorphosis at different rates. A classification of lesions based on morphological appearance may help disease prognosis and examination of the effect of the lesion on disease symptoms, and provide new opportunities for targeted therapies in order to prevent or treat endometriosis. Surgical removal of endometriotic lesions resulted in a high incidence of recurrence. Spontaneous endometriosis developed in control baboons in the absence of inoculation suggesting that repetitive surgical procedures alone can induce the spontaneous evolution of the chronic disease. Although lesion excision/ablation may have short-term benefits (e.g. prior to an IVF cycle in subfertile women), for long-term relief of symptoms perhaps medical therapy is more effective than surgical therapy.
Figures



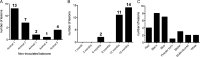
References
-
- Abbott JA, Hawe J, Clayton RD, Garry R. The effects and effectiveness of laparoscopic excision of endometriosis: a prospective study with 2–5 year follow-up. Hum Reprod. 2003;18:1922–1927. - PubMed
-
- American Fertility Society. Revised American Fertility Society classification of endometriosis 1985. Fertil Steril. 1985;43:351–352. - PubMed
-
- American Fertility Society. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–821. - PubMed
-
- Brosens I. Endometriosis rediscovered? Hum Reprod. 2004;19:1679–1680. author reply 1680–1671. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

