Medical management of Cushing's disease: what is the future?
- PMID: 22674211
- PMCID: PMC3443360
- DOI: 10.1007/s11102-012-0397-5
Medical management of Cushing's disease: what is the future?
Abstract
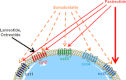
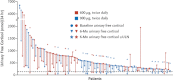
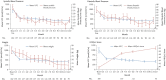
Cushing's disease (CD) is caused by a corticotroph, adrenocorticotropic-hormone (ACTH)-secreting pituitary adenoma resulting in significant morbidity and mortality. Transsphenoidal surgery is the initial treatment of choice in almost all cases. Remission rates for microadenomas are good at 65-90 % (with an experienced neurosurgeon) but remission rates are much lower for macroadenomas. However, even after postoperative remission, recurrence rates are high and can be seen up to decades after an initial diagnosis. Repeat surgery or radiation can be useful in these cases, although both have clear limitations with respect to efficacy and/or side effects. Hence, there is a clear unmet need for an effective medical treatment. Currently, most drugs act by inhibiting steroidogenesis in the adrenal glands. Most is known about the effects of ketoconazole and metyrapone. While effective, access to ketoconazole and metyrapone is limited in many countries, experience with long-term use is limited, and side effects can be significant. Recent studies have suggested a role for a pituitary-directed therapy with new multireceptor ligand somatostatin analogs (e.g., pasireotide, recently approved in Europe for treatment of CD), second-generation dopamine agonists, or a combination of both. Mifepristone (a glucocorticoid receptor antagonist) is another promising drug, recently approved by the FDA for treatment of hyperglycemia associated with Cushing's syndrome. We review available medical treatments for CD with a focus on the two most recent compounds referenced above. Our aim is to expand awareness of current research, and the possibilities afforded by available medical treatments for this mesmerizing, but often frightful disease.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

