The mesenchymal stem cell marker CD248 (endosialin) is a negative regulator of bone formation in mice
- PMID: 22674221
- PMCID: PMC4209224
- DOI: 10.1002/art.34556
The mesenchymal stem cell marker CD248 (endosialin) is a negative regulator of bone formation in mice
Abstract
Objective: CD248 (tumor endothelial marker 1/endosialin) is found on stromal cells and is highly expressed during malignancy and inflammation. Studies have shown a reduction in inflammatory arthritis in CD248-knockout (CD248(-/-) ) mice. The aim of the present study was to investigate the functional effect of genetic deletion of CD248 on bone mass.
Methods: Western blotting, polymerase chain reaction, and immunofluorescence were used to investigate the expression of CD248 in humans and mice. Micro-computed tomography and the 3-point bending test were used to measure bone parameters and mechanical properties of the tibiae of 10-week-old wild-type (WT) or CD248(-/-) mice. Human and mouse primary osteoblasts were cultured in medium containing 10 mM β-glycerophosphate and 50 μg/ml ascorbic acid to induce mineralization, and then treated with platelet-derived growth factor BB (PDGF-BB). The mineral apposition rate in vivo was calculated by identifying newly formed bone via calcein labeling.
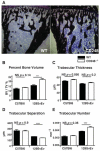
Results: Expression of CD248 was seen in human and mouse osteoblasts, but not osteoclasts. CD248(-/-) mouse tibiae had higher bone mass and superior mechanical properties (increased load required to cause fracture) compared to WT mice. Primary osteoblasts from CD248(-/-) mice induced increased mineralization in vitro and produced increased bone over 7 days in vivo. There was no decrease in bone mineralization and no increase in proliferation of osteoblasts in response to stimulation with PDGF-BB, which could be attributed to a defect in PDGF signal transduction in the CD248(-/-) mice.
Conclusion: There is an unmet clinical need to address rheumatoid arthritis-associated bone loss. Genetic deletion of CD248 in mice results in high bone mass due to increased osteoblast-mediated bone formation, suggesting that targeting CD248 in rheumatoid arthritis may have the effect of increasing bone mass in addition to the previously reported effect of reducing inflammation.
Copyright © 2012 by the American College of Rheumatology.
Figures





References
-
- Bagley RG, Honma N, Weber W, Boutin P, Rouleau C, Shankara S, et al. Endosialin/TEM1/CD248 is a pericyte marker of embryonic and tumor neovascularisation. Microvasc Res. 2008;76:180–8. - PubMed
-
- Smith SW, Eardley KS, Croft A, Nwosu J, Howie AJ, Cockwell P, et al. CD248+ stromal cells are associated with progressive chronic kidney disease. Kidney Int. 2011;80:199–207. - PubMed
-
- MacFadyen J, Savage K, Wienke D, Isacke CM. Endosialin is expressed on stromal fibroblasts and CNS pericytes in mouse embryos and is downregulated during development. Gene Expr Patterns. 2007;7:363–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

