State-dependent spike and local field synchronization between motor cortex and substantia nigra in hemiparkinsonian rats
- PMID: 22674263
- PMCID: PMC3423905
- DOI: 10.1523/JNEUROSCI.0943-12.2012
State-dependent spike and local field synchronization between motor cortex and substantia nigra in hemiparkinsonian rats
Abstract
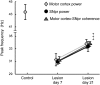
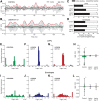
Excessive beta frequency oscillatory and synchronized activity has been reported in the basal ganglia of parkinsonian patients and animal models of the disease. To gain insight into processes underlying this activity, this study explores relationships between oscillatory activity in motor cortex and basal ganglia output in behaving rats after dopamine cell lesion. During inattentive rest, 7 d after lesion, increases in motor cortex-substantia nigra pars reticulata (SNpr) coherence emerged in the 8-25 Hz range, with significant increases in local field potential (LFP) power in SNpr but not motor cortex. In contrast, during treadmill walking, marked increases in both motor cortex and SNpr LFP power, as well as coherence, emerged in the 25-40 Hz band with a peak frequency at 30-35 Hz. Spike-triggered waveform averages showed that 77% of SNpr neurons, 77% of putative cortical interneurons, and 44% of putative pyramidal neurons were significantly phase-locked to the increased cortical LFP activity in the 25-40 Hz range. Although the mean lag between cortical and SNpr LFPs fluctuated around zero, SNpr neurons phase-locked to cortical LFP oscillations fired, on average, 17 ms after synchronized spiking in motor cortex. High coherence between LFP oscillations in cortex and SNpr supports the view that cortical activity facilitates entrainment and synchronization of activity in basal ganglia after loss of dopamine. However, the dramatic increases in cortical power and relative timing of phase-locked spiking in these areas suggest that additional processes help shape the frequency-specific tuning of the basal ganglia-thalamocortical network during ongoing motor activity.
Figures



References
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. - PubMed
-
- Alonso-Frech F, Zamarbide I, Alegre M, Rodríguez-Oroz MC, Guridi J, Manrique M, Valencia M, Artieda J, Obeso JA. Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson's disease. Brain. 2006;129:1748–1757. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
