HuR inhibits apoptosis by amplifying Akt signaling through a positive feedback loop
- PMID: 22674407
- PMCID: PMC3443509
- DOI: 10.1002/jcp.24120
HuR inhibits apoptosis by amplifying Akt signaling through a positive feedback loop
Abstract
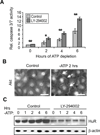
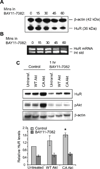
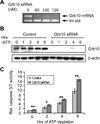
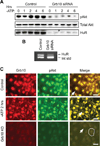
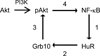
Human antigen R (HuR) is a post-transcriptional regulator of gene expression that plays a key role in stabilizing mRNAs during cellular stress, leading to enhanced survival. HuR expression is tightly regulated through multiple transcription and post-transcriptional controls. Although HuR is known to stabilize a subset of mRNAs involved in cell survival, its role in the survival pathway of PI3-kinase/Akt signaling is unclear. Here, we show that in renal proximal tubule cells, HuR performs a central role in cell survival by amplifying Akt signaling in a positive feedback loop. Key to this feedback loop is HuR-mediated stabilization of mRNA encoding Grb10, an adaptor protein whose expression is critical for Akt activation. Stimulation of Akt by interaction with Grb10 then activates NF-κB, which further enhances HuR mRNA and protein expression. This feedback loop is active in unstressed cells, but its effects are increased during stress. Therefore, this study demonstrates a central role for HuR in Akt signaling and reveals a mechanism by which modest changes in HuR levels below or above normal may be amplified, potentially resulting in cell death or cellular transformation.
Copyright © 2012 Wiley Periodicals, Inc.
Figures

Similar articles
-
Expression of the RNA-stabilizing protein HuR in ischemia-reperfusion injury of rat kidney.Am J Physiol Renal Physiol. 2009 Jul;297(1):F95-F105. doi: 10.1152/ajprenal.90632.2008. Epub 2009 May 6. Am J Physiol Renal Physiol. 2009. PMID: 19420108 Free PMC article.
-
NF-kappaB activates transcription of the RNA-binding factor HuR, via PI3K-AKT signaling, to promote gastric tumorigenesis.Gastroenterology. 2008 Dec;135(6):2030-42, 2042.e1-3. doi: 10.1053/j.gastro.2008.08.009. Epub 2008 Aug 20. Gastroenterology. 2008. PMID: 18824170
-
Concurrent binding and modifications of AUF1 and HuR mediate the pH-responsive stabilization of phosphoenolpyruvate carboxykinase mRNA in kidney cells.Am J Physiol Renal Physiol. 2012 Dec 1;303(11):F1545-54. doi: 10.1152/ajprenal.00400.2012. Epub 2012 Sep 26. Am J Physiol Renal Physiol. 2012. PMID: 23019227 Free PMC article.
-
Functional interplay between RNA-binding protein HuR and microRNAs.Curr Protein Pept Sci. 2012 Jun;13(4):372-9. doi: 10.2174/138920312801619394. Curr Protein Pept Sci. 2012. PMID: 22708488 Free PMC article. Review.
-
Regulation of the mRNA-binding protein HuR by posttranslational modification: spotlight on phosphorylation.Curr Protein Pept Sci. 2012 Jun;13(4):380-90. doi: 10.2174/138920312801619439. Curr Protein Pept Sci. 2012. PMID: 22708484 Review.
Cited by
-
A HuR/TGF-β1 feedback circuit regulates airway remodeling in airway smooth muscle cells.Respir Res. 2016 Sep 22;17(1):117. doi: 10.1186/s12931-016-0437-1. Respir Res. 2016. PMID: 27658983 Free PMC article.
-
Hu Antigen R (HuR) Protein Structure, Function and Regulation in Hepatobiliary Tumors.Cancers (Basel). 2022 May 27;14(11):2666. doi: 10.3390/cancers14112666. Cancers (Basel). 2022. PMID: 35681645 Free PMC article. Review.
-
Adaptive and maladaptive expression of the mRNA regulatory protein HuR.World J Biol Chem. 2013 Nov 26;4(4):111-8. doi: 10.4331/wjbc.v4.i4.111. World J Biol Chem. 2013. PMID: 24340134 Free PMC article. Review.
-
TLR9 signaling repressed tumor suppressor miR-7 expression through up-regulation of HuR in human lung cancer cells.Cancer Cell Int. 2013 Sep 3;13(1):90. doi: 10.1186/1475-2867-13-90. Cancer Cell Int. 2013. PMID: 24004462 Free PMC article.
-
Essential Roles of RNA-binding Protein HuR in Activation of Hepatic Stellate Cells Induced by Transforming Growth Factor-β1.Sci Rep. 2016 Feb 25;6:22141. doi: 10.1038/srep22141. Sci Rep. 2016. PMID: 26912347 Free PMC article.
References
-
- Abdelmohsen K, Lal A, Kim HH, Gorospe M. Posttranscriptional orchestration of an anti-apoptotic program by HuR. Cell Cycle. 2007;6(11):1288–1292. - PubMed
-
- Andreucci M, Michael A, Kramers C, Park KM, Chen A, Matthaeus T, Alessandrini A, Haq S, Force T, Bonventre JV. Renal ischemia/reperfusion and ATP depletion/repletion in LLC-PK(1) cells result in phosphorylation of FKHR and FKHRL1. Kidney Int. 2003;64(4):1189–1198. - PubMed
-
- Atasoy U, Watson J, Patel D, Keene JD. ELAV protein HuA (HuR) can redistribute between nucleus and cytoplasm and is upregulated during serum stimulation and T cell activation. J Cell Sci. 1998;111(Pt 21):3145–3156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

