Regulation of early xenopus embryogenesis by Smad ubiquitination regulatory factor 2
- PMID: 22674516
- PMCID: PMC3399951
- DOI: 10.1002/dvdy.23811
Regulation of early xenopus embryogenesis by Smad ubiquitination regulatory factor 2
Abstract
Background: Smad ubiquitination regulatory factor (Smurf) 1 and 2 are E3 ubiquitin ligases originally identified as inhibitors of transforming growth factor beta signaling and are shown to modulate multiple cellular activities. The roles of Smurfs in vertebrate embryogenesis, however, are not completely understood.
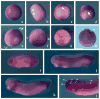
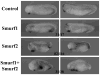
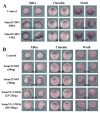
Results: Here we investigate the function of Smurf2 during early Xenopus development. We show that distinctly from Smurf1, overexpression of Smurf2 in presumptive mesoderm interfered with mesoderm induction and caused axial defects, whereas knockdown of Smurf2 with antisense morpholino oligonucleotides resulted in expansion of the mesoderm. These results imply that Smurf2 may modulate nodal-mediated mesodermal induction. Consistently, ventral expression of Smurf2 induced a partial secondary axis with head structures. In the ectoderm, Smurf2 resembled Smurf1 in controlling neural and epidermal marker expression and influencing head formation. Smurf1, but not Smurf2, additionally affected neural tube closure. Interestingly, both Smurfs could enhance as well as repress neural crest markers, implying that they modulate their targets dynamically during neural plate border specification.
Conclusion: Our data demonstrate that Smurf1 and Smurf2 have overlapping and distinct functionalities during early frog embryogenesis; collectively, they regulate ectodermal and mesodermal induction and patterning to ensure normal development of Xenopus embryos.
Copyright © 2012 Wiley Periodicals, Inc.
Figures







References
-
- Amaravadi LS, Neff AW, Sleeman JP, Smith RC. Autonomous neural axis formation by ectopic expression of the protooncogene c-ski. Dev Biol. 1997;192:392–404. - PubMed
-
- Aybar MJ, Mayor R. Early induction of neural crest cells: lessons learned from frog, fish and chick. Curr Opin Genet Dev. 2002;12:452–458. - PubMed
-
- Baker CV, Bronner-Fraser M. The origins of the neural crest. Part I: embryonic induction. Mech Dev. 1997;69:3–11. - PubMed
-
- Barembaum M, Bronner-Fraser M. Early steps in neural crest specification. Semin Cell Dev Biol. 2005;16:642–646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

