Totally implantable venous power ports of the forearm and the chest: initial clinical experience with port devices approved for high-pressure injections
- PMID: 22674705
- PMCID: PMC3500819
- DOI: 10.1259/bjr/33224341
Totally implantable venous power ports of the forearm and the chest: initial clinical experience with port devices approved for high-pressure injections
Abstract
Objectives: To evaluate the technical success, clinical outcome and safety of percutaneously placed totally implantable venous power ports (TIVPPs) approved for high-pressure injections, and to analyse their value for arterial phase CT scans.
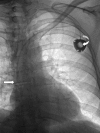
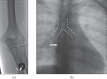
Methods: Retrospectively, we identified 204 patients who underwent TIVPP implantation in the forearm (n=152) or chest (n=52) between November 2009 and May 2011. Implantation via an upper arm (forearm port, FP) or subclavian vein (chest port, CP) was performed under sonographic and fluoroscopic guidance. Complications were evaluated following the standards of the Society of Interventional Radiology. Power injections via TIVPPs were analysed, focusing on adequate functioning and catheter's tip location after injection. Feasibility of automatic bolus triggering, peak injection pressure and arterial phase aortic enhancement were evaluated and compared with 50 patients who had had power injections via classic peripheral cannulas.
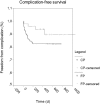
Results: Technical success was 100%. Procedure-related complications were not observed. Catheter-related thrombosis was diagnosed in 15 of 152 FPs (9.9%, 0.02/100 catheter days) and in 1 of 52 CPs (1.9%, 0.002/100 catheter days) (p<0.05). Infectious complications were diagnosed in 9 of 152 FPs (5.9%, 0.014/100 catheter days) and in 2 of 52 CPs (3.8%, 0.003/100 catheter days) (p>0.05). Arterial bolus triggering succeeded in all attempts; the mean injection pressure was 213.8 psi. Aortic enhancement did not significantly differ between injections via cannulas and TIVPPs (p>0.05).
Conclusions: TIVPPs can be implanted with high technical success rates, and are associated with low rates of complications if implanted with sonographic and fluoroscopic guidance. Power injections via TIVPPs are safe and result in satisfying arterial contrast. Conventional ports should be replaced by TIVPPs.
Figures
Similar articles
-
Femoral placement of totally implantable venous power ports as an alternative implantation site for patients with central vein occlusions.Support Care Cancer. 2014 Feb;22(2):383-7. doi: 10.1007/s00520-013-1984-3. Epub 2013 Sep 24. Support Care Cancer. 2014. PMID: 24061782
-
Feasibility of power contrast injections and bolus triggering during CT scans in oncologic patients with totally implantable venous access ports of the forearm.Acta Radiol. 2011 Feb 1;52(1):41-7. doi: 10.1258/ar.2010.100238. Acta Radiol. 2011. PMID: 21498324
-
Peripherally placed totally implantable venous-access port systems of the forearm: clinical experience in 763 consecutive patients.Cardiovasc Intervent Radiol. 2010 Dec;33(6):1159-67. doi: 10.1007/s00270-010-9854-6. Epub 2010 Apr 23. Cardiovasc Intervent Radiol. 2010. PMID: 20414657
-
Complications after percutaneous placement of totally implantable venous access ports in the forearm.Clin Radiol. 2012 Nov;67(11):1101-7. doi: 10.1016/j.crad.2012.03.007. Epub 2012 May 13. Clin Radiol. 2012. PMID: 22583951 Review.
-
Comparison of complications between peripheral arm ports and central chest ports: A meta-analysis.J Adv Nurs. 2018 Nov;74(11):2484-2496. doi: 10.1111/jan.13766. Epub 2018 Jul 25. J Adv Nurs. 2018. PMID: 29917252 Review.
Cited by
-
Improved Computed Tomography Contrast Injection Rates Through Implantable Chest Power Ports.J Comput Assist Tomogr. 2020 Nov/Dec;44(6):911-913. doi: 10.1097/RCT.0000000000001048. J Comput Assist Tomogr. 2020. PMID: 32976270 Free PMC article.
-
Current situation regarding central venous port implantation procedures and complications: a questionnaire-based survey of 11,693 implantations in Japan.Int J Clin Oncol. 2016 Dec;21(6):1172-1182. doi: 10.1007/s10147-016-1003-z. Epub 2016 Jun 21. Int J Clin Oncol. 2016. PMID: 27324107
-
Incidence of and risk factors for totally implantable vascular access device complications in patients with gastric cancer: A retrospective analysis.Mol Clin Oncol. 2019 Oct;11(4):343-348. doi: 10.3892/mco.2019.1897. Epub 2019 Jul 15. Mol Clin Oncol. 2019. PMID: 31475061 Free PMC article.
-
Femoral placement of totally implantable venous power ports as an alternative implantation site for patients with central vein occlusions.Support Care Cancer. 2014 Feb;22(2):383-7. doi: 10.1007/s00520-013-1984-3. Epub 2013 Sep 24. Support Care Cancer. 2014. PMID: 24061782
-
Upper-Extremity Deep Vein Thrombosis in Patients With Breast Cancer With Chest Versus Arm Central Venous Port Catheters.Breast Cancer (Auckl). 2018 Apr 20;12:1178223418771909. doi: 10.1177/1178223418771909. eCollection 2018. Breast Cancer (Auckl). 2018. PMID: 29881287 Free PMC article.
References
-
- Kock HJ, Pietsch M, Krause U, Wilke H, Eigler FW. Implantable vascular access systems: experience in 1500 patients with totally implanted central venous port systems. World J Surg 1998;22:12–16 - PubMed
-
- Kawamura J, Nagayama S, Nomura A, Itami A, Okabe H, Sato S, et al. Long-term outcomes of peripheral arm ports implanted in patients with colorectal cancer. Int J Clin Oncol 2008;13:349–54 - PubMed
-
- Goltz JP, Scholl A, Ritter CO, Wittenberg G, Hahn D, Kickuth R. Peripherally placed totally implantable venous-access port systems of the forearm: clinical experience in 763 consecutive patients. Cardiovasc Intervent Radiol 2010;33:1159–67 - PubMed
-
- Marcy PY, Magne N, Castadot P, Bailet C, Macchiavello JC, Namer M, et al. Radiological and surgical placement of port devices: a 4-year institutional analysis of procedure performance, quality of life and cost in breast cancer patients. Breast Cancer Res Treat 2005;92:61–7 - PubMed
-
- Kuriakose P, Colon-Otero G, Paz-Fumagalli R. Risk of deep venous thrombosis associated with chest versus arm central venous subcutaneous port catheters: a 5-year single-institution retrospective study. J Vasc Interv Radiol 2002;13:179–84 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

