M-type potassium channels modulate Schaffer collateral-CA1 glutamatergic synaptic transmission
- PMID: 22674722
- PMCID: PMC3476642
- DOI: 10.1113/jphysiol.2012.235820
M-type potassium channels modulate Schaffer collateral-CA1 glutamatergic synaptic transmission
Abstract
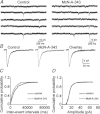
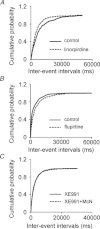
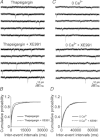
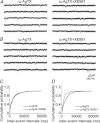
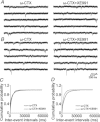
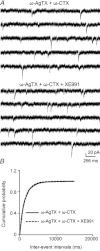
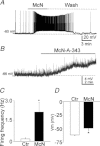
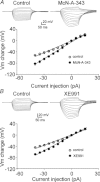
Previous studies have suggested that muscarinic receptor activation modulates glutamatergic transmission. M-type potassium channels mediate the effects of muscarinic activation in the hippocampus, and it has been proposed that they modulate glutamatergic synaptic transmission. We tested whether M1 muscarinic receptor activation enhances glutamatergic synaptic transmission via the inhibition of the M-type potassium channels that are present in Schaffer collateral axons and terminals. Miniature excitatory postsynaptic currents (mEPSCs) were recorded from CA1 pyramidal neurons. The M1 receptor agonist, NcN-A-343, increased the frequency of mEPSCs, but did not alter their amplitude. The M-channel blocker XE991 and its analogue linopirdine also increased the frequency of mEPSCs. Flupirtine, which opens M-channels, had the opposite effect. XE991 did not enhance mEPSCs frequency in a calcium-free external medium. Blocking P/Q- and N-type calcium channels abolished the effect of XE991 on mEPSCs. These data suggested that the inhibition of M-channels increases presynaptic calcium-dependent glutamate release in CA1 pyramidal neurons. The effects of these agents on the membrane potentials of presynaptic CA3 pyramidal neurons were studied using current clamp recordings; activation of M1 receptors and blocking M-channels depolarized neurons and increased burst firing. The input resistance of CA3 neurons was increased by the application of McN-A-343 and XE991; these effects were consistent with the closure of M-channels. Muscarinic activation inhibits M-channels in CA3 pyramidal neurons and its efferents – Schaffer collateral, which causes the depolarization, activates voltage-gated calcium channels, and ultimately elevates the intracellular calcium concentration to increase the release of glutamate on CA1 pyramidal neurons.
Figures


Similar articles
-
Kv7/KCNQ/M-channels in rat glutamatergic hippocampal axons and their role in regulation of excitability and transmitter release.J Physiol. 2006 Oct 1;576(Pt 1):235-56. doi: 10.1113/jphysiol.2006.111336. Epub 2006 Jul 13. J Physiol. 2006. PMID: 16840518 Free PMC article.
-
Adenosine A1 antagonism increases specific synaptic forms of glutamate release during anoxia, revealing a unique source of excitation.Hippocampus. 1996;6(3):213-24. doi: 10.1002/(SICI)1098-1063(1996)6:3<213::AID-HIPO1>3.0.CO;2-O. Hippocampus. 1996. PMID: 8841822
-
Potentiation of glutamatergic synaptic input to supraoptic neurons by presynaptic nicotinic receptors.Am J Physiol Regul Integr Comp Physiol. 2001 Oct;281(4):R1105-13. doi: 10.1152/ajpregu.2001.281.4.R1105. Am J Physiol Regul Integr Comp Physiol. 2001. PMID: 11557616
-
Neural KCNQ (Kv7) channels.Br J Pharmacol. 2009 Apr;156(8):1185-95. doi: 10.1111/j.1476-5381.2009.00111.x. Epub 2009 Mar 9. Br J Pharmacol. 2009. PMID: 19298256 Free PMC article. Review.
-
Muscarinic acetylcholine receptors (mAChRs) in the nervous system: some functions and mechanisms.J Mol Neurosci. 2010 Jul;41(3):340-6. doi: 10.1007/s12031-010-9377-2. Epub 2010 May 6. J Mol Neurosci. 2010. PMID: 20446119 Review.
Cited by
-
Diverse impact of acute and long-term extracellular proteolytic activity on plasticity of neuronal excitability.Front Cell Neurosci. 2015 Aug 10;9:313. doi: 10.3389/fncel.2015.00313. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26321914 Free PMC article. Review.
-
Modulation of Kv7 channels and excitability in the brain.Cell Mol Life Sci. 2017 Feb;74(3):495-508. doi: 10.1007/s00018-016-2359-y. Epub 2016 Sep 19. Cell Mol Life Sci. 2017. PMID: 27645822 Free PMC article. Review.
-
Glycolytic lactate production supports status epilepticus in experimental animals.Ann Clin Transl Neurol. 2023 Oct;10(10):1873-1884. doi: 10.1002/acn3.51881. Epub 2023 Aug 25. Ann Clin Transl Neurol. 2023. PMID: 37632130 Free PMC article.
-
Potassium and calcium channels in different nerve cells act as therapeutic targets in neurological disorders.Neural Regen Res. 2025 May 1;20(5):1258-1276. doi: 10.4103/NRR.NRR-D-23-01766. Epub 2024 Jun 3. Neural Regen Res. 2025. PMID: 38845230 Free PMC article.
-
A novel therapeutic approach for treatment of catamenial epilepsy.Neurobiol Dis. 2018 Mar;111:127-137. doi: 10.1016/j.nbd.2017.12.009. Epub 2017 Dec 21. Neurobiol Dis. 2018. PMID: 29274741 Free PMC article.
References
-
- Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P, Jentsch TJ, Steinlein OK. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279:403–406. - PubMed
-
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

