Analysis of factors affecting Ca(2+)-dependent inactivation dynamics of L-type Ca(2+) current of cardiac myocytes in pulmonary vein of rabbit
- PMID: 22674726
- PMCID: PMC3477751
- DOI: 10.1113/jphysiol.2012.229203
Analysis of factors affecting Ca(2+)-dependent inactivation dynamics of L-type Ca(2+) current of cardiac myocytes in pulmonary vein of rabbit
Abstract
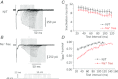
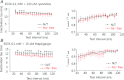
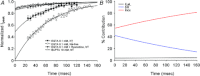
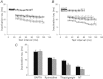
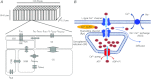
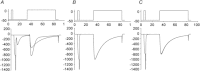
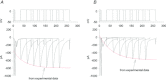
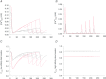
L-type Ca(2+) channels (ICaLs) are inactivated by an increase in intracellular [Ca(2+)], known as Ca(2+)-dependent inactivation (CDI). CDI is also induced by Ca(2+) released from the sarcoplasmic reticulum (SR), known as release-dependent inhibition (RDI). As both CDI and RDI occur in the junctional subsarcolemmal nanospace (JSS), we investigated which factors are involved within the JSS using isolated cardiac myocytes from the main pulmonary vein of the rabbit. Using the whole-cell patch clamp technique, RDI was readily observed with the application of a pre-pulse followed by a test pulse, during which the ICaLs exhibited a decrease in peak current amplitude and a slower inactivation. A fast acting Ca(2+) chelator, 1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA), abolished this effect. As the time interval between the pre-pulse and test pulse increased, the ICaLs exhibited greater recovery and the RDI was relieved. Inhibition of the ryanodine receptor (RyR) or the SR Ca(2+)-ATPase (SERCA) greatly attenuated RDI and facilitated ICaL recovery. Removal of extracellular Na(+),which inhibits the Na(+)-Ca(2+) exchange (Incx), greatly enhanced RDI and slowed ICaL recovery, suggesting that Incx critically controls the [Ca(2+)] in the JSS. We incorporated the Ca(2+)-binding kinetics of the ICaL into a previously published computational model. By assuming two Ca(2+)-binding sites in the ICaL, of which one is of low-affinity with fast kinetics and the other is of high-affinity with slower kinetics, the new model was able to successfully reproduce RDI and its regulation by Incx. The model suggests that Incx accelerates Ca(2+) removal from the JSS to downregulate CDI and attenuates SR Ca(2+) refilling. The model may be useful to elucidate complex mechanisms involved in excitation–contraction coupling in myocytes.
Figures




Similar articles
-
Microdomain [Ca²⁺] near ryanodine receptors as reported by L-type Ca²⁺ and Na+/Ca²⁺ exchange currents.J Physiol. 2011 May 15;589(Pt 10):2569-83. doi: 10.1113/jphysiol.2010.202663. Epub 2011 Mar 8. J Physiol. 2011. PMID: 21486798 Free PMC article.
-
Theoretical study of L-type Ca(2+) current inactivation kinetics during action potential repolarization and early afterdepolarizations.J Physiol. 2012 Sep 15;590(18):4465-81. doi: 10.1113/jphysiol.2012.231886. Epub 2012 May 14. J Physiol. 2012. PMID: 22586219 Free PMC article.
-
Calmodulin kinase II accelerates L-type Ca2+ current recovery from inactivation and compensates for the direct inhibitory effect of [Ca2+]i in rat ventricular myocytes.J Physiol. 2006 Jul 15;574(Pt 2):509-18. doi: 10.1113/jphysiol.2006.109199. Epub 2006 Apr 20. J Physiol. 2006. PMID: 16627565 Free PMC article.
-
Inactivation of L-type calcium channels in cardiomyocytes. Experimental and theoretical approaches.Gen Physiol Biophys. 2003 Dec;22(4):441-54. Gen Physiol Biophys. 2003. PMID: 15113117 Review.
-
Modulation of L-type calcium current kinetics by sarcoplasmic reticulum calcium release in ferret isolated right ventricular myocytes.Can J Cardiol. 1998 Feb;14(2):263-72. Can J Cardiol. 1998. PMID: 9520864 Review.
Cited by
-
What can modelling provide to cardiac physiology?J Physiol. 2012 Sep 15;590(18):4401-2. doi: 10.1113/jphysiol.2012.242578. J Physiol. 2012. PMID: 22988258 Free PMC article. No abstract available.
-
Impaired Inactivation of L-Type Ca2+ Current as a Potential Mechanism for Variable Arrhythmogenic Liability of HERG K+ Channel Blocking Drugs.PLoS One. 2016 Mar 1;11(3):e0149198. doi: 10.1371/journal.pone.0149198. eCollection 2016. PLoS One. 2016. PMID: 26930604 Free PMC article.
-
L-type Ca2+ channel recovery from inactivation in rabbit atrial myocytes.Physiol Rep. 2022 Mar;10(5):e15222. doi: 10.14814/phy2.15222. Physiol Rep. 2022. PMID: 35274829 Free PMC article.
References
-
- Anderson ME. Ca2+-dependent regulation of cardiac L-type Ca2+ channels: is a unifying mechanism at hand? J Mol Cell Cardiol. 2001;33:639–650. - PubMed
-
- Balke CW, Wier WG. Ryanodine does not affect calcium current in guinea pig ventricular myocytes in which Ca2+ is buffered. Circ Res. 1991;68:897–902. - PubMed
-
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Boston: Kluwer; 2001.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

