Efficient solvent-free hydrogenation of ketones over flame-prepared bimetallic Pt-Pd/ZrO(2) catalysts
- PMID: 22674738
- PMCID: PMC3796871
- DOI: 10.1002/cssc.201200126
Efficient solvent-free hydrogenation of ketones over flame-prepared bimetallic Pt-Pd/ZrO(2) catalysts
Abstract
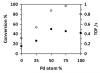
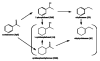
Named and flamed: Bimetallic Pt-Pd/ZrO(2) catalysts with different Pt/Pd atomic ratios and high dispersion of the metal nanoparticles are prepared by a single-step flame-spray pyrolysis. The catalysts show excellent activity and tunable product selectivity for the solvent-free hydrogenation of the ketone model compounds cyclopentanone and acetophenone.
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures




Similar articles
-
Microwave-assisted versatile hydrogenation of carbonyl compounds using supported metal nanoparticles.Org Biomol Chem. 2009 Dec 7;7(23):4821-4. doi: 10.1039/b913695a. Epub 2009 Oct 2. Org Biomol Chem. 2009. PMID: 19907769
-
Polysilane-supported Pd and Pt nanoparticles as efficient catalysts for organic synthesis.Chem Commun (Camb). 2006 Nov 4;(41):4297-9. doi: 10.1039/b610241g. Epub 2006 Sep 5. Chem Commun (Camb). 2006. PMID: 17047846
-
Development of Nickel- and Magnetite-Promoted Carbonized Cellulose Bead-Supported Bimetallic Pd-Pt Catalysts for Hydrogenation of Chlorate Ions in Aqueous Solution.Int J Mol Sci. 2021 Oct 31;22(21):11846. doi: 10.3390/ijms222111846. Int J Mol Sci. 2021. PMID: 34769280 Free PMC article.
-
Homogeneous palladium-catalyzed asymmetric hydrogenation.Chem Soc Rev. 2013 Jan 21;42(2):497-511. doi: 10.1039/c2cs35333d. Chem Soc Rev. 2013. PMID: 23138972 Review.
-
Enhancing the catalytic and electrocatalytic properties of Pt-based catalysts by forming bimetallic nanocrystals with Pd.Chem Soc Rev. 2012 Dec 21;41(24):8035-49. doi: 10.1039/c2cs35173k. Chem Soc Rev. 2012. PMID: 23080521 Review.
Cited by
-
Solvent-Free Hydrogenation of Squalene Using Parts per Million Levels of Palladium Supported on Carbon Nanotubes: Shift from Batch Reactor to Continuous-Flow System.ChemSusChem. 2022 Oct 10;15(19):e202200916. doi: 10.1002/cssc.202200916. Epub 2022 Aug 25. ChemSusChem. 2022. PMID: 35880580 Free PMC article.
References
-
- Dresselhaus MS, Thomas IL. Nature. 2001;414:332–337. - PubMed
-
- Potocnik J. Science. 2007;315:810–811. - PubMed
-
- Huber GW, Iborra S, Corma A. Chem. Rev. 2006;106:4044–4098. - PubMed
-
- Zhao C, Kou Y, Lemonidou AA, Li XB, Lercher JA. Angew. Chem. Int. Ed. 2009;48:3987–3990. - PubMed
-
- Wang Y, Yao J, Li HR, Su DS, Antonietti M. J. Am. Chem. Soc. 2011;133:2362–2365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

