Revealing the role of phospholipase Cβ3 in the regulation of VEGF-induced vascular permeability
- PMID: 22674805
- PMCID: PMC3447777
- DOI: 10.1182/blood-2012-03-417824
Revealing the role of phospholipase Cβ3 in the regulation of VEGF-induced vascular permeability
Abstract
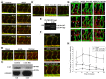
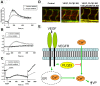
VEGF induces vascular permeability (VP) in ischemic diseases and cancer, leading to many pathophysiological consequences. The molecular mechanisms by which VEGF acts to induce hyperpermeability are poorly understood and in vivo models that easily facilitate real-time, genetic studies of VP do not exist. In the present study, we report a heat-inducible VEGF transgenic zebrafish (Danio rerio) model through which VP can be monitored in real time. Using this approach with morpholino-mediated gene knock-down and knockout mice, we describe a novel role of phospholipase Cβ3 as a negative regulator of VEGF-mediated VP by regulating intracellular Ca2+ release. Our results suggest an important effect of PLCβ3 on VP and provide a new model with which to identify genetic regulators of VP crucial to several disease processes.
Figures


Comment in
-
Zebrafish lead the way in control of vascular permeability.Blood. 2012 Sep 13;120(11):2162-4. doi: 10.1182/blood-2012-07-439182. Blood. 2012. PMID: 22977082 No abstract available.
References
-
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–985. - PubMed
-
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306–1309. - PubMed
-
- Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380(6573):435–439. - PubMed
-
- Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380(6573):439–442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

