Developmental expression of the cellular prion protein (PrP(C) ) in bovine embryos
- PMID: 22674901
- PMCID: PMC3389575
- DOI: 10.1002/mrd.22057
Developmental expression of the cellular prion protein (PrP(C) ) in bovine embryos
Abstract
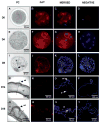
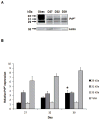
The mammalian cellular prion protein (PrP(C) ) is a highly conserved glycoprotein that may undergo conversion into a conformationally altered isoform (scrapie prion protein or PrP(Sc) ), widely believed to be the pathogenic agent of transmissible spongiform encephalopathies (TSEs). Although much is known about PrP(Sc) conversion and its role in TSEs, the normal function of PrP(C) has not been elucidated. In adult mammals, PrP(C) is most abundant in the central nervous tissue, with intermediate levels in the intestine and heart, and lower levels in the pancreas and liver. PrP(C) is expressed during neurogenesis throughout development, and it has recently been proposed that PrP(C) participates in neural cell differentiation during embryogenesis. In order to establish the developmental timing and to address the cell-specific expression of PrP(C) during mammalian development, we examined PrP(C) expression in bovine gametes and embryos through gestation Day 39. Our data revealed differential levels of Prnp mRNA at Days 4 and 18 in pre-attachment embryos. PrP(C) was detected in the developing central and peripheral nervous systems in Day-27, 32-, and -39 embryos. PrP(C) was particularly expressed in differentiated neural cells located in the marginal regions of the central nervous system, but was absent from mitotically active, periventricular areas. Moreover, a PrP(C) cell-specific pattern of expression was detected in non-nervous tissues, including liver and mesonephros, during these stages. The potential participation of PrP(C) in neural cell differentiation is supported by its specific expression in differentiated states of neurogenesis.
Copyright © 2012 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
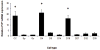



References
-
- Aguzzi A, Heppner FL, Heikenwalder M, Prinz M, Mertz K, Seeger H, Glatzel M. Immune system and peripheral nerves in propagation of prions to CNS. Br Med Bull. 2003;66:141–159. - PubMed
-
- Bounhar Y, Zhang Y, Goodyer CG, LeBlanc A. Prion protein protects human neurons against Bax-mediated apoptosis. J Biol Chem. 2001;276:39145–39149. - PubMed
-
- Bueler H, Fischer M, Lang Y, Bluethmann H, Lipp H-P, DeArmond SJ, Prusiner SB, Aguet M, Weissmann C. Normal development and behavior of mice lacking the normal cell-surface PrP protein. Nature. 1992;356:577–582. - PubMed
-
- Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, Weissman C. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

