Kaposi's sarcoma-associated herpesvirus ORF54/dUTPase downregulates a ligand for the NK activating receptor NKp44
- PMID: 22674989
- PMCID: PMC3421743
- DOI: 10.1128/JVI.00252-12
Kaposi's sarcoma-associated herpesvirus ORF54/dUTPase downregulates a ligand for the NK activating receptor NKp44
Abstract
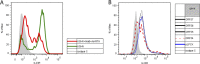
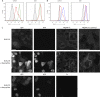
Kaposi's sarcoma-associated herpesvirus (KSHV) establishes long-term latent infection in humans and can cause cancers in endothelial and B cells. A functioning immune system is vital for restricting viral proliferation and preventing KSHV-dependent neoplasms. While natural killer (NK) lymphocytes are known to target virus-infected cells for destruction, their importance in the anti-KSHV immune response is not currently understood. Activating receptors on NK cells recognize ligands on target cells, including the uncharacterized ligand(s) for NKp44, termed NKp44L. Here we demonstrate that several NK ligands are affected when KSHV-infected cells are induced to enter the lytic program. We performed a screen of most of the known KSHV genes and found that the product of the ORF54 gene could downregulate NKp44L. The ORF54-encoded protein is a dUTPase; however, dUTPase activity is neither necessary nor sufficient for the downregulation of NKp44L. In addition, we find that ORF54 can also target proteins of the cytokine receptor family and the mechanism of downregulation involves perturbation of membrane protein trafficking. The ORF54-related proteins of other human herpesviruses do not possess this activity, suggesting that the KSHV homolog has evolved a novel immunoregulatory function and that the NKp44-NKp44L signaling pathway contributes to antiviral immunity.
Figures
References
-
- Ambroziak JA, et al. 1995. Herpes-like sequences in HIV-infected and uninfected Kaposi's sarcoma patients. Science 268:582–583 - PubMed
-
- Barabas O, Pongracz V, Kovari J, Wilmanns M, Vertessy BG. 2004. Structural insights into the catalytic mechanism of phosphate ester hydrolysis by dUTPase. J. Biol. Chem. 279:42907–42915 - PubMed
-
- Bergman AC, Nyman PO, Larsson G. 1998. Kinetic properties and stereospecificity of the monomeric dUTPase from herpes simplex virus type 1. FEBS Lett. 441:327–330 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

