Cot/tpl2-MKK1/2-Erk1/2 controls mTORC1-mediated mRNA translation in Toll-like receptor-activated macrophages
- PMID: 22675026
- PMCID: PMC3408424
- DOI: 10.1091/mbc.E12-02-0135
Cot/tpl2-MKK1/2-Erk1/2 controls mTORC1-mediated mRNA translation in Toll-like receptor-activated macrophages
Abstract
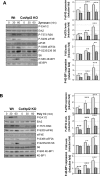
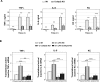
Cot/tpl2 is the only MAP3K that activates MKK1/2-Erk1/2 in Toll-like receptor-activated macrophages. Here we show that Cot/tpl2 regulates RSK, S6 ribosomal protein, and 4E-BP phosphorylation after stimulation of bone marrow-derived macrophages with lipopolysaccharide (LPS), poly I:C, or zymosan. The dissociation of the 4E-BP-eIF4E complex, a key event in the cap-dependent mRNA translation initiation, is dramatically reduced in LPS-stimulated Cot/tpl2-knockout (KO) macrophages versus LPS-stimulated wild-type (Wt) macrophages. Accordingly, after LPS activation, increased cap-dependent translation is observed in Wt macrophages but not in Cot/tpl2 KO macrophages. In agreement with these data, Cot/tpl2 increases the polysomal recruitment of the 5´ TOP eEF1α and eEF2 mRNAs, as well as of inflammatory mediator gene-encoding mRNAs, such as tumor necrosis factor α (TNFα), interleukin-6 (IL-6), and KC in LPS-stimulated macrophages. In addition, Cot/tpl2 deficiency also reduces total TNFα, IL-6, and KC mRNA expression in LPS-stimulated macrophages, which is concomitant with a decrease in their mRNA half-lives. Macrophages require rapid fine control of translation to provide an accurate and not self-damaging response to host infection, and our data show that Cot/tpl2 controls inflammatory mediator gene-encoding mRNA translation in Toll-like receptor-activated macrophages.
Figures





References
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. - PubMed
-
- Anderson P. Post-transcriptional control of cytokine production. Nat Immunol. 2008;9:353–359. - PubMed
-
- Anderson P. Post-transcriptional regulons coordinate the initiation and resolution of inflammation. Nat Rev Immunol. 2010;10:24–35. - PubMed
-
- Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9:747–758. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

