Myosin 1E coordinates actin assembly and cargo trafficking during clathrin-mediated endocytosis
- PMID: 22675027
- PMCID: PMC3408416
- DOI: 10.1091/mbc.E11-04-0383
Myosin 1E coordinates actin assembly and cargo trafficking during clathrin-mediated endocytosis
Abstract
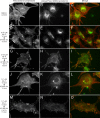
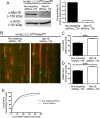
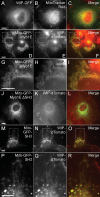
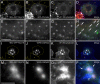
Myosin 1E (Myo1E) is recruited to sites of clathrin-mediated endocytosis coincident with a burst of actin assembly. The recruitment dynamics and lifetime of Myo1E are similar to those of tagged actin polymerization regulatory proteins. Like inhibition of actin assembly, depletion of Myo1E causes reduced transferrin endocytosis and a significant delay in transferrin trafficking to perinuclear compartments, demonstrating an integral role for Myo1E in these actin-mediated steps. Mistargeting of GFP-Myo1E or its src-homology 3 domain to mitochondria results in appearance of WIP, WIRE, N-WASP, and actin filaments at the mitochondria, providing evidence for Myo1E's role in actin assembly regulation. These results suggest for mammalian cells, similar to budding yeast, interdependence in the recruitment of type I myosins, WIP/WIRE, and N-WASP to endocytic sites for Arp2/3 complex activation to assemble F-actin as endocytic vesicles are being formed.
Figures




Similar articles
-
Myosin 1E interacts with synaptojanin-1 and dynamin and is involved in endocytosis.FEBS Lett. 2007 Feb 20;581(4):644-50. doi: 10.1016/j.febslet.2007.01.021. Epub 2007 Jan 18. FEBS Lett. 2007. PMID: 17257598 Free PMC article.
-
Type I myosins anchor actin assembly to the plasma membrane during clathrin-mediated endocytosis.J Cell Biol. 2019 Apr 1;218(4):1138-1147. doi: 10.1083/jcb.201810005. Epub 2019 Jan 18. J Cell Biol. 2019. PMID: 30659101 Free PMC article.
-
An Engineered Minimal WASP-Myosin Fusion Protein Reveals Essential Functions for Endocytosis.Dev Cell. 2015 Nov 9;35(3):281-94. doi: 10.1016/j.devcel.2015.10.007. Dev Cell. 2015. PMID: 26555049 Free PMC article.
-
Coupling actin dynamics and membrane dynamics during endocytosis.Curr Opin Cell Biol. 2002 Feb;14(1):76-81. doi: 10.1016/s0955-0674(01)00297-6. Curr Opin Cell Biol. 2002. PMID: 11792548 Review.
-
Local actin polymerization during endocytic carrier formation.Biochem Soc Trans. 2018 Jun 19;46(3):565-576. doi: 10.1042/BST20170355. Epub 2018 Apr 20. Biochem Soc Trans. 2018. PMID: 29678956 Review.
Cited by
-
Myo1e overexpression in lung adenocarcinoma is associated with increased risk of mortality.Sci Rep. 2023 Mar 13;13(1):4107. doi: 10.1038/s41598-023-30765-y. Sci Rep. 2023. PMID: 36914720 Free PMC article.
-
Tail domains of myosin-1e regulate phosphatidylinositol signaling and F-actin polymerization at the ventral layer of podosomes.Mol Biol Cell. 2019 Mar 1;30(5):622-635. doi: 10.1091/mbc.E18-06-0398. Epub 2019 Jan 2. Mol Biol Cell. 2019. PMID: 30601698 Free PMC article.
-
Actomyosin-driven force patterning controls endocytosis at the immune synapse.Nat Commun. 2019 Jun 28;10(1):2870. doi: 10.1038/s41467-019-10751-7. Nat Commun. 2019. PMID: 31253773 Free PMC article.
-
Capping protein-controlled actin polymerization shapes lipid membranes.Nat Commun. 2018 Apr 24;9(1):1630. doi: 10.1038/s41467-018-03918-1. Nat Commun. 2018. PMID: 29691404 Free PMC article.
-
Epsin deficiency impairs endocytosis by stalling the actin-dependent invagination of endocytic clathrin-coated pits.Elife. 2014 Aug 13;3:e03311. doi: 10.7554/eLife.03311. Elife. 2014. PMID: 25122462 Free PMC article.
References
-
- Benesch S, Polo S, Lai FP, Anderson KI, Stradal TE, Wehland J, Rottner K. N-WASP deficiency impairs EGF internalization and actin assembly at clathrin-coated pits. J Cell Sci. 2005;118:3103–3115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

