Contrasting enantioselective DNA preference: chiral helical macrocyclic lanthanide complex binding to DNA
- PMID: 22675072
- PMCID: PMC3439914
- DOI: 10.1093/nar/gks524
Contrasting enantioselective DNA preference: chiral helical macrocyclic lanthanide complex binding to DNA
Abstract
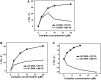
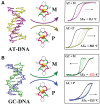
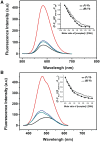
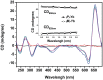
There is great interest in design and synthesis of small molecules which selectively target specific genes to inhibit biological functions in which particular DNA structures participate. Among these studies, chiral recognition has been received much attention because more evidences have shown that conversions of the chirality and diverse conformations of DNA are involved in a series of important life events. Here, we report that a pair of chiral helical macrocyclic lanthanide (III) complexes, (M)-Yb[L(SSSSSS)](3+) and (P)-Yb[L(RRRRRR)](3+), can enantioselectively bind to B-form DNA and show remarkably contrasting effects on GC-rich and AT-rich DNA. Neither of them can influence non-B-form DNA, nor quadruplex DNA stability. Our results clearly show that P-enantiomer stabilizes both poly(dG-dC)(2) and poly(dA-dT)(2) while M-enantiomer stabilizes poly(dA-dT)(2), however, destabilizes poly(dG-dC)(2). To our knowledge, this is the best example of chiral metal compounds with such contrasting preference on GC- and AT-DNA. Ligand selectively stabilizing or destabilizing DNA can interfere with protein-DNA interactions and potentially affect many crucial biological processes, such as DNA replication, transcription and repair. As such, bearing these unique capabilities, the chiral compounds reported here may shed light on the design of novel enantiomers targeting specific DNA with both sequence and conformation preference.
Figures


Similar articles
-
Thermodynamics of nucleic acid "shape readout" by an aminosugar.Biochemistry. 2011 Oct 25;50(42):9088-113. doi: 10.1021/bi201077h. Epub 2011 Oct 3. Biochemistry. 2011. PMID: 21863895 Free PMC article.
-
Enantioselective binding of chiral 1,14-dimethyl[5]helicene-spermine ligands with B- and Z-DNA.Bioorg Med Chem. 2013 Oct 1;21(19):6063-8. doi: 10.1016/j.bmc.2013.07.022. Epub 2013 Jul 18. Bioorg Med Chem. 2013. PMID: 23969037
-
Lanthanide complexes of the chiral hexaaza macrocycle and its meso-type isomer: solvent-controlled helicity inversion.Inorg Chem. 2007 Sep 17;46(19):7923-34. doi: 10.1021/ic700831z. Epub 2007 Aug 17. Inorg Chem. 2007. PMID: 17705368
-
Conformational variability of poly(dA-dT).poly(dA-dT) and some other deoxyribonucleic acids includes a novel type of double helix.J Biomol Struct Dyn. 1985 Aug;3(1):67-83. doi: 10.1080/07391102.1985.10508399. J Biomol Struct Dyn. 1985. PMID: 3917211 Review.
-
New cleft-like molecules and macrocycles from phosphonate substituted spirobisindanol.Molecules. 2008 Mar 20;13(3):678-700. doi: 10.3390/molecules13030678. Molecules. 2008. PMID: 18463570 Free PMC article. Review.
Cited by
-
Luminescent Lanthanides in Biorelated Applications: From Molecules to Nanoparticles and Diagnostic Probes to Therapeutics.Chem Rev. 2025 Feb 26;125(4):2269-2370. doi: 10.1021/acs.chemrev.4c00615. Epub 2025 Feb 17. Chem Rev. 2025. PMID: 39960048 Free PMC article. Review.
-
Recognizing and stabilizing miR-21 by chiral ruthenium(II) complexes.BMC Chem. 2020 Apr 3;14(1):26. doi: 10.1186/s13065-020-00672-8. eCollection 2020 Dec. BMC Chem. 2020. PMID: 32266333 Free PMC article.
-
SOD mimetic activity and antiproliferative properties of a novel tetra nuclear copper (II) complex.J Biol Inorg Chem. 2015 Dec;20(8):1287-98. doi: 10.1007/s00775-015-1307-x. Epub 2015 Nov 7. J Biol Inorg Chem. 2015. PMID: 26547749
-
DNA Recognition by a Novel Bis-Intercalator, Potent Anticancer Drug XR5944.Curr Top Med Chem. 2015;15(14):1385-97. doi: 10.2174/1568026615666150413155608. Curr Top Med Chem. 2015. PMID: 25866279 Free PMC article. Review.
-
Imine- and Amine-Type Macrocycles Derived from Chiral Diamines and Aromatic Dialdehydes.Molecules. 2022 Jun 25;27(13):4097. doi: 10.3390/molecules27134097. Molecules. 2022. PMID: 35807342 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

