Individual maize chromosomes in the C(3) plant oat can increase bundle sheath cell size and vein density
- PMID: 22675083
- PMCID: PMC3425187
- DOI: 10.1104/pp.112.200584
Individual maize chromosomes in the C(3) plant oat can increase bundle sheath cell size and vein density
Abstract
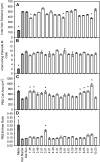
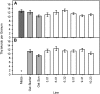
C(4) photosynthesis has evolved in at least 66 lineages within the angiosperms and involves alterations to the biochemistry, cell biology, and development of leaves. The characteristic "Kranz" anatomy of most C(4) leaves was discovered in the 1890s, but the genetic basis of these traits remains poorly defined. Oat × maize addition lines allow the effects of individual maize (Zea mays; C(4)) chromosomes to be investigated in an oat (Avena sativa; C(3)) genetic background. Here, we have determined the extent to which maize chromosomes can introduce C(4) characteristics into oat and have associated any C(4)-like changes with specific maize chromosomes. While there is no indication of a simultaneous change to C(4) biochemistry, leaf anatomy, and ultrastructure in any of the oat × maize addition lines, the C(3) oat leaf can be modified at multiple levels. Maize genes encoding phosphoenolpyruvate carboxylase, pyruvate, orthophosphate dikinase, and the 2'-oxoglutarate/malate transporter are expressed in oat and generate transcripts of the correct size. Three maize chromosomes independently cause increases in vein density, and maize chromosome 3 results in larger bundle sheath cells with increased cell wall lipid deposition in oat leaves. These data provide proof of principle that aspects of C(4) biology could be integrated into leaves of C(3) crops.
Figures




References
-
- Agarie S, Miura A, Sumikura R, Tsukamoto S, Nose A, Arima S, Matsuoka M, Miyao-Tokutomi M. (2002) Overexpression of C4 PEPC caused O2-insensitive photosynthesis in transgenic rice plants. Plant Sci 162: 257–265
-
- Brown NJ, Sullivan JA, Gray JC. (2005) Light and plastid signals regulate the expression of the pea plastocyanin gene through a common region at the 5′ end of the coding region. Plant J 43: 541–552 - PubMed
-
- Brown WV. (1958) Leaf anatomy in grass systematics. Bot Gaz 119: 170–178
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

