All in the Family: How the APPs Regulate Neurogenesis
- PMID: 22675290
- PMCID: PMC3366480
- DOI: 10.3389/fnins.2012.00081
All in the Family: How the APPs Regulate Neurogenesis
Abstract
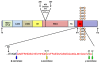
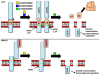
Recent intriguing evidence suggests that metabolites of amyloid precursor protein (APP), mutated in familial forms of Alzheimer's disease (AD), play critical roles in developmental and postnatal neurogenesis. Of note is soluble APPα (sAPPα) that regulates neural progenitor cell proliferation. The APP family encompasses a group of ubiquitously expressed and evolutionarily conserved, type I transmembrane glycoproteins, whose functions have yet to be fully elucidated. APP can undergo proteolytic cleavage by mutually exclusive pathways. The subtle structural differences between metabolites generated in the different pathways, as well as their equilibrium, may be crucial for neuronal function. The implications of this new body of evidence are significant. Miscleavage of APP would readily impact developmental and postnatal neurogenesis, which might contribute to cognitive deficits characterizing Alzheimer's disease. This review will discuss the implications of the role of the APP family in neurogenesis for neuronal development, cognitive function, and brain disorders that compromise learning and memory, such as AD.
Keywords: Alzheimer’s disease; aging; amyloid precursor protein; learning and memory; neurogenesis; neuronal plasticity.
Figures

References
-
- Ayuso-Sacido A., Moliterno J. A., Kratovac S., Kapoor G. S., O’Rourke D. M., Holland E. C., Garcia-Verdugo J. M., Roy N. S., Boockvar J. A. (2010). Activated EGFR signaling increases proliferation, survival, and migration and blocks neuronal differentiation in post-natal neural stem cells. J. Neurooncol. 97, 323–33710.1007/s11060-009-0035-x - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

