High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson's disease
- PMID: 22675296
- PMCID: PMC3366347
- DOI: 10.3389/fnhum.2012.00155
High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson's disease
Abstract
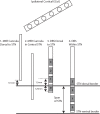
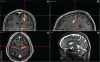
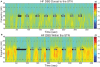
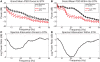
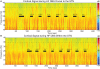
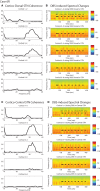
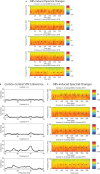
Parkinson's disease (PD) is marked by excessive synchronous activity in the beta (8-35 Hz) band throughout the cortico-basal ganglia network. The optimal location of high frequency deep brain stimulation (HF DBS) within the subthalamic nucleus (STN) region and the location of maximal beta hypersynchrony are currently matters of debate. Additionally, the effect of STN HF DBS on neural synchrony in functionally connected regions of motor cortex is unknown and is of great interest. Scalp EEG studies demonstrated that stimulation of the STN can activate motor cortex antidromically, but the spatial specificity of this effect has not been examined. The present study examined the effect of STN HF DBS on neural synchrony within the cortico-basal ganglia network in patients with PD. We measured local field potentials dorsal to and within the STN of PD patients, and additionally in the motor cortex in a subset of these patients. We used diffusion tensor imaging (DTI) to guide the placement of subdural cortical surface electrodes over the DTI-identified origin of the hyperdirect pathway (HDP) between motor cortex and the STN. The results demonstrated that local beta power was attenuated during HF DBS both dorsal to and within the STN. The degree of attenuation was monotonic with increased DBS voltages in both locations, but this voltage-dependent effect was greater in the central STN than dorsal to the STN (p < 0.05). Cortical signals over the estimated origin of the HDP also demonstrated attenuation of beta hypersynchrony during DBS dorsal to or within STN, whereas signals from non-specific regions of motor cortex were not attenuated. The spatially-specific suppression of beta synchrony in the motor cortex support the hypothesis that DBS may treat Parkinsonism by reducing excessive synchrony in the functionally connected sensorimotor network.
Keywords: Parkinson's disease; beta rhythm; deep brain stimulation (DBS); electrocorticography (ECoG); intracranial EEG; motor cortex; subthalamic nucleus; synchrony.
Figures



References
-
- Albin R. L., Young A. B., Penney J. B. (1989). The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375 - PubMed
-
- Ashby P., Paradiso G., Saint-Cyr J. A., Chen R., Lang A. E., Lozano A. M. (2001). Potentials recorded at the scalp by stimulation near the human subthalamic nucleus. Clin. Neurophysiol. 112, 431–437 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

