Current standards in the treatment of chronic hepatitis C
- PMID: 22675406
- PMCID: PMC3364529
- DOI: 10.3238/arztebl.2012.0352
Current standards in the treatment of chronic hepatitis C
Abstract
Background: In Germany, 400 000 to 500 000 people are chronically infected with the hepatitis C virus (HCV), 70% to 80% of them with HCV genotype 1. Combined treatment with peginterferon-alfa and ribavirin leads to a sustained virologic response (SVR) in 40% to 50% of patients with genotype 1 and 70% to 80% of patients with genotypes 2 and 3. The HCV protease inhibitors boceprevir and telaprevir were approved for clinical use in Germany in 2011.
Methods: Selective literature review.
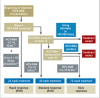
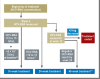
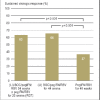
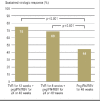
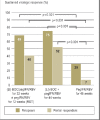
Results: Treatment with peginterferon and ribavirin is recommended for a variable length of time depending on the HCV genotype (24 to 72 weeks for genotype 1, 16 to 48 weeks for genotypes 2 and 3), the baseline HCV-RNA concentration (greater or less than 600 000 to 800 000 IU/mL), and the decline in HCV-RNA concentration after 4 and 12 weeks of treatment. Either boceprevir or telaprevir is given in addition to peginterferon and ribavirin. In the approval studies, these triple combinations were shown to yield higher SVR rates than dual treatment for genotype 1 (66% to 75% versus 37% to 44%). If there is a favorable early decline in HCV-RNA, the treatment can be shortened to 24 to 28 weeks in 44% to 65% of patients with genotype 1. The SVR rates in genotype 1 patients who failed previous dual therapy were 69% to 88% for prior relapsers, 52% to 59% for partial responders, and 33% for null responders. Triple combination therapy is associated with new adverse events.
Conclusion: Individualized treatment durations are recommended for the treatment of chronic hepatitis C with peginterferon and ribavirin. Triple therapy in combination with either boceprevir or telaprevir leads to a higher rate of SVR both in previously untreated genotype 1 patients and in those who have failed prior antiviral treatment.\
Figures


Comment in
-
Early assessment of benefits.Dtsch Arztebl Int. 2012 Nov;109(44):755; author reply 755-6. doi: 10.3238/arztebl.2012.0755a. Epub 2012 Nov 2. Dtsch Arztebl Int. 2012. PMID: 23189113 Free PMC article. No abstract available.
References
-
- Sarrazin C, Berg T, Ross RS, et al. [Prophylaxis, diagnosis and therapy of hepatitis C virus (HCV) infection: the German guidelines on the management of HCV infection] Z Gastroenterol. 2010;48:289–351. - PubMed
-
- Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:35–46. - PubMed
-
- Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:35–50. - PubMed
-
- Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. - PubMed
-
- Zeuzem S, Hultcrantz R, Bourliere M, et al. Peginterferon alfa-2b plus ribavirin for treatment of chronic hepatitis C in previously untreated patients infected with HCV genotypes 2 or 3. J Hepatol. 2004;40:993–999. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

