Melanoma chemotherapy leads to the selection of ABCB5-expressing cells
- PMID: 22675422
- PMCID: PMC3360047
- DOI: 10.1371/journal.pone.0036762
Melanoma chemotherapy leads to the selection of ABCB5-expressing cells
Abstract
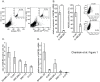
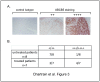
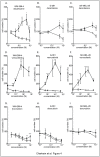
Metastatic melanoma is the most aggressive skin cancer. Recently, phenotypically distinct subpopulations of tumor cells were identified. Among them, ABCB5-expressing cells were proposed to display an enhanced tumorigenicity with stem cell-like properties. In addition, ABCB5(+) cells are thought to participate to chemoresistance through a potential efflux function of ABCB5. Nevertheless, the fate of these cells upon drugs that are used in melanoma chemotherapy remains to be clarified. Here we explored the effect of anti-melanoma treatments on the ABCB5-expressing cells. Using a melanoma xenograft model (WM266-4), we observed in vivo that ABCB5-expressing cells are enriched after a temozolomide treatment that induces a significant tumor regression. These results were further confirmed in a preliminary study conducted on clinical samples from patients that received dacarbazine. In vitro, we showed that ABCB5-expressing cells selectively survive when exposed to dacarbazine, the reference treatment of metastatic melanoma, but also to vemurafenib, a new inhibitor of the mutated kinase V600E BRAF and other various chemotherapeutic drugs. Our results show that anti-melanoma chemotherapy might participate to the chemoresistance acquisition by selecting tumor cell subpopulations expressing ABCB5. This is of particular importance in understanding the relapses observed after anti-melanoma treatments and reinforces the interest of ABCB5 and ABCB5-expressing cells as potential therapeutic targets in melanoma.
Conflict of interest statement
Figures



Similar articles
-
Differential expression of ABCB5 in BRAF inhibitor-resistant melanoma cell lines.BMC Cancer. 2018 Jun 22;18(1):675. doi: 10.1186/s12885-018-4583-3. BMC Cancer. 2018. PMID: 29929490 Free PMC article.
-
ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma.Cancer Res. 2005 May 15;65(10):4320-33. doi: 10.1158/0008-5472.CAN-04-3327. Cancer Res. 2005. PMID: 15899824
-
The helicase HAGE prevents interferon-α-induced PML expression in ABCB5+ malignant melanoma-initiating cells by promoting the expression of SOCS1.Cell Death Dis. 2014 Feb 13;5(2):e1061. doi: 10.1038/cddis.2014.29. Cell Death Dis. 2014. PMID: 24525737 Free PMC article.
-
Vemurafenib in patients with BRAF V600E mutation-positive advanced melanoma.Clin Ther. 2012 Jul;34(7):1474-86. doi: 10.1016/j.clinthera.2012.06.009. Epub 2012 Jun 27. Clin Ther. 2012. PMID: 22742884 Review.
-
[Drug resistance of malignant melanoma. Mechanisms and possible modulation].Hautarzt. 1994 Oct;45(10):678-84. doi: 10.1007/s001050050149. Hautarzt. 1994. PMID: 8002335 Review. German.
Cited by
-
Parthenolide reduces the frequency of ABCB5-positive cells and clonogenic capacity of melanoma cells from anchorage independent melanospheres.Cancer Biol Ther. 2013 Feb;14(2):135-45. doi: 10.4161/cbt.22952. Epub 2012 Nov 28. Cancer Biol Ther. 2013. PMID: 23192276 Free PMC article.
-
Natural Hemp-Ginger Extract and Its Biological and Therapeutic Efficacy.Molecules. 2022 Nov 9;27(22):7694. doi: 10.3390/molecules27227694. Molecules. 2022. PMID: 36431795 Free PMC article.
-
Growth Hormone Upregulates Melanocyte-Inducing Transcription Factor Expression and Activity via JAK2-STAT5 and SRC Signaling in GH Receptor-Positive Human Melanoma.Cancers (Basel). 2019 Sep 12;11(9):1352. doi: 10.3390/cancers11091352. Cancers (Basel). 2019. PMID: 31547367 Free PMC article.
-
Deep-proteome mapping of WM-266-4 human metastatic melanoma cells: From oncogenic addiction to druggable targets.PLoS One. 2017 Feb 3;12(2):e0171512. doi: 10.1371/journal.pone.0171512. eCollection 2017. PLoS One. 2017. PMID: 28158294 Free PMC article.
-
IGF-1 contributes to the expansion of melanoma-initiating cells through an epithelial-mesenchymal transition process.Oncotarget. 2016 Dec 13;7(50):82511-82527. doi: 10.18632/oncotarget.12733. Oncotarget. 2016. PMID: 27764776 Free PMC article.
References
-
- Garbe C, Leiter U. Melanoma epidemiology and trends. Clin Dermatol. 2009;27:3–9. - PubMed
-
- Abolhoda A, Wilson AE, Ross H, Danenberg PV, Burt M, et al. Rapid activation of MDR1 gene expression in human metastatic sarcoma after in vivo exposure to doxorubicin. Clin Cancer Res. 1999;5:3352–3356. - PubMed
-
- Jilaveanu LB, Aziz SA, Kluger HM. Chemotherapy and biologic therapies for melanoma: do they work? Clin Dermatol. 2009;27:614–625. - PubMed
-
- Garbe C, Peris K, Hauschild A, Saiag P, Middleton M, et al. Diagnosis and treatment of melanoma: European consensus-based interdisciplinary guideline. Eur J Cancer. 2010;46:270–283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

