Networked T cell death following macrophage infection by Mycobacterium tuberculosis
- PMID: 22675566
- PMCID: PMC3366923
- DOI: 10.1371/journal.pone.0038488
Networked T cell death following macrophage infection by Mycobacterium tuberculosis
Abstract
Background: Depletion of T cells following infection by Mycobacterium tuberculosis (Mtb) impairs disease resolution, and interferes with clinical test performance that relies on cell-mediated immunity. A number of mechanisms contribute to this T cell suppression, such as activation-induced death and trafficking of T cells out of the peripheral circulation and into the diseased lungs. The extent to which Mtb infection of human macrophages affects T cell viability however, is not well characterised.
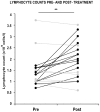
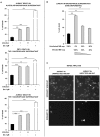
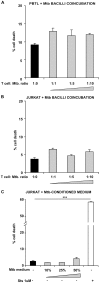
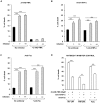
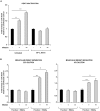
Methodology/principal findings: We found that lymphopenia (<1.5 × 10(9) cells/l) was prevalent among culture-positive tuberculosis patients, and lymphocyte counts significantly improved post-therapy. We previously reported that Mtb-infected human macrophages resulted in death of infected and uninfected bystander macrophages. In the current study, we sought to examine the influence of infected human alveolar macrophages on T cells. We infected primary human alveolar macrophages (the primary host cell for Mtb) or PMA-differentiated THP-1 cells with Mtb H37Ra, then prepared cell-free supernatants. The supernatants of Mtb-infected macrophages caused dose-dependent, caspase-dependent, T cell apoptosis. This toxic effect of infected macrophage secreted factors did not require TNF-α or Fas. The supernatant cytotoxic signal(s) were heat-labile and greater than 50 kDa in molecular size. Although ESAT-6 was toxic to T cells, other Mtb-secreted factors tested did not influence T cell viability; nor did macrophage-free Mtb bacilli or broth from Mtb cultures. Furthermore, supernatants from Mycobacterium bovis Bacille de Calmette et Guerin (BCG)- infected macrophages also elicited T cell death suggesting that ESAT-6 itself, although cytotoxic, was not the principal mediator of T cell death in our system.
Conclusions: Mtb-Infected macrophages secrete heat-labile factors that are toxic to T cells, and may contribute to the immunosuppression seen in tuberculosis as well as interfere with microbial eradication in the granuloma.
Conflict of interest statement
Figures

References
-
- Davoudi S, Rasoolinegad M, Younesian M, Hajiabdolbaghi M, Soudbakhsh A, et al. CD4+ cell counts in patients with different clinical manifestations of tuberculosis. Brazilian Journal of Infectious Diseases. 2008;12:483–486. - PubMed
-
- Jones BE, Oo MM, Taikwel EK, Qian D, Kumar A, et al. CD4 Cell Counts in Human Immunodeficiency Virus-Negative Patients with Tuberculosis. Clinical Infectious Diseases. 1997;24:988–991. - PubMed
-
- Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, et al. Autophagy Is a Defense Mechanism Inhibiting BCG and Mycobacterium tuberculosis Survival in Infected Macrophages. Cell. 2004;119:753–766. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

