Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease
- PMID: 22675658
- PMCID: PMC3367542
- DOI: 10.1101/cshperspect.a006262
Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease
Abstract
Progressive cerebral deposition of the amyloid β-protein (Aβ) in brain regions serving memory and cognition is an invariant and defining feature of Alzheimer disease. A highly similar but less robust process accompanies brain aging in many nondemented humans, lower primates, and some other mammals. The discovery of Aβ as the subunit of the amyloid fibrils in meningocerebral blood vessels and parenchymal plaques has led to innumerable studies of its biochemistry and potential cytotoxic properties. Here we will review the discovery of Aβ, numerous aspects of its complex biochemistry, and current attempts to understand how a range of Aβ assemblies, including soluble oligomers and insoluble fibrils, may precipitate and promote neuronal and glial alterations that underlie the development of dementia. Although the role of Aβ as a key molecular factor in the etiology of Alzheimer disease remains controversial, clinical trials of amyloid-lowering agents, reviewed elsewhere in this book, are poised to resolve the question of its pathogenic primacy.
Figures

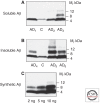
References
-
- Abraham CR, Selkoe DJ, Potter H 1988. Immunochemical identification of the serine protease inhibitor, α1-antichymotrypsin in the brain amyloid deposits of Alzheimer’s disease. Cell 52: 487–501 - PubMed
-
- Allsop D, Landon M, Kidd M 1983. The isolation and amino acid composition of senile plaque core protein. Brain Res 259: 348–352 - PubMed
-
- Barman A, Taves W, Prabhakar R 2009. Insights into the mechanism of methionine oxidation catalyzed by metal (Cu2+, Zn2+, and Fe3+)-amyloid beta (Aβ) peptide complexes: A computational study. J Comput Chem 30: 1405–1413 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
