An open-label, multicenter evaluation of the long-term safety and efficacy of risperidone in adolescents with schizophrenia
- PMID: 22676070
- PMCID: PMC3489792
- DOI: 10.1186/1753-2000-6-23
An open-label, multicenter evaluation of the long-term safety and efficacy of risperidone in adolescents with schizophrenia
Abstract
Background: Data on the long-term efficacy, safety, and tolerability of risperidone in adolescents with schizophrenia are limited. The objective of this study was to evaluate the efficacy and safety of maintenance risperidone treatment in adolescents with schizophrenia.
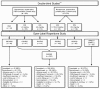
Methods: This open-label study of adolescents aged 13 to 17 years with schizophrenia was a single extension study of two short-term double-blind risperidone studies and also enrolled subjects directly in open-label risperidone treatment. The risperidone dose was flexible and ranged from 2 to 6 mg/day. Most subjects enrolled for 6 months; a subset enrolled for 12 months. Assessment tools included the Positive and Negative Syndrome Scale total and factor scores, Clinical Global Impressions, Children's Global Assessment Scale, adverse event (AE) monitoring, vital signs, laboratory testing, and extrapyramidal symptom rating scales.
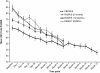
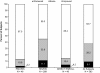
Results: A total of 390 subjects were enrolled; 48 subjects had received placebo in a previous double-blind study; 292 subjects had received risperidone as part of their participation in one of two previous controlled studies; and 50 subjects were enrolled directly for this study. A total of 279 subjects enrolled for 6 months of treatment, and 111 subjects enrolled for 12 months of treatment. Overall, 264 (67.7%) subjects completed this study: 209 of the 279 subjects (75%) in the 6-month group and 55 of the 111 subjects (50%) in the 12-month group. The median mode dose was 3.8 mg/day. At 6 months, all three groups experienced improvement from open-label baseline in symptoms of schizophrenia as well as general assessments of global functioning. Improvements were generally maintained for the duration of treatment. The most common AEs (≥10% of subjects) were somnolence, headache, weight increase, hypertonia, insomnia, tremor, and psychosis. Potentially prolactin-related AEs (PPAEs) were reported by 36 (9%) subjects. The AE profile in this study was qualitatively similar to those of other studies in adult subjects with schizophrenia and in other psychiatric studies of risperidone in pediatric populations.
Conclusions: Risperidone maintenance treatment in adolescents over 6 to 12 months was well tolerated, consistent with related studies in this clinical population, and associated with continued efficacy.
Clinical trials: ClinicalTrials.gov registration number: NCT00246285 http://clinicaltrials.gov/ct2/show/NCT00246285?term=NCT00246285&rank=1.
Figures

Similar articles
-
A 6-week, randomized, double-blind, placebo-controlled study of the efficacy and safety of risperidone in adolescents with schizophrenia.J Child Adolesc Psychopharmacol. 2009 Dec;19(6):611-21. doi: 10.1089/cap.2008.0144. J Child Adolesc Psychopharmacol. 2009. PMID: 20035579 Clinical Trial.
-
An open-label extension trial of risperidone monotherapy in the treatment of bipolar I disorder.Int Clin Psychopharmacol. 2006 Jan;21(1):11-20. doi: 10.1097/01.yic.0000177017.41558.fc. Int Clin Psychopharmacol. 2006. PMID: 16317312 Clinical Trial.
-
Switching to Lurasidone following 12 months of treatment with Risperidone: results of a 6-month, open-label study.BMC Psychiatry. 2020 May 5;20(1):199. doi: 10.1186/s12888-020-02523-1. BMC Psychiatry. 2020. PMID: 32370778 Free PMC article. Clinical Trial.
-
[Antipsychotics in bipolar disorders].Encephale. 2004 Sep-Oct;30(5):417-24. doi: 10.1016/s0013-7006(04)95456-5. Encephale. 2004. PMID: 15627046 Review. French.
-
Paliperidone extended release: a review of its use in the management of schizophrenia.Drugs. 2010 Jul 9;70(10):1295-317. doi: 10.2165/11204840-000000000-00000. Drugs. 2010. PMID: 20568835 Review.
Cited by
-
Prolactin-related adverse events and change in prolactin levels in pediatric patients given antipsychotics for schizophrenia and schizophrenia spectrum disorders: A systematic review.BMC Pediatr. 2016 Nov 9;16(1):181. doi: 10.1186/s12887-016-0710-y. BMC Pediatr. 2016. PMID: 27825323 Free PMC article.
-
Alternative delivery systems for agents to treat acute agitation: progress to date.Drugs. 2013 Nov;73(16):1783-92. doi: 10.1007/s40265-013-0130-3. Drugs. 2013. PMID: 24151084 Review.
-
Long-Term Safety and Efficacy of Blonanserin Oral Tablet in Adolescents with Schizophrenia: A 52-Week, Multicenter, Open-Label Extension Study.J Child Adolesc Psychopharmacol. 2022 Feb;32(1):24-35. doi: 10.1089/cap.2021.0058. Epub 2021 Oct 5. J Child Adolesc Psychopharmacol. 2022. PMID: 34612724 Free PMC article.
-
Promises and Pitfalls of NMDA Receptor Antagonists in Treating Violent Aggression.Front Behav Neurosci. 2022 Jun 21;16:938044. doi: 10.3389/fnbeh.2022.938044. eCollection 2022. Front Behav Neurosci. 2022. PMID: 35801096 Free PMC article.
-
Antipsychotics in children and adolescents with schizophrenia: a systematic review and meta-analysis.Indian J Pharmacol. 2013 Sep-Oct;45(5):439-46. doi: 10.4103/0253-7613.117720. Indian J Pharmacol. 2013. PMID: 24130376 Free PMC article.
References
-
- Asarnow JR, Tompson MC, Goldstein MJ. Childhood-onset schizophrenia: a followup study. Schizophr Bull. 1994;20:599–617. - PubMed
-
- Remschmidt H, Theisen FM. Schizophrenia and related disorders in children and adolescents. J Neural Transm Suppl. 2005. pp. 121–141. - PubMed
-
- Remschmidt HE, Schulz E, Martin M, Warnke A, Trott GE. Childhood-onset schizophrenia: history of the concept and recent studies. Schizophr Bull. 1994;20:727–745. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

