Randomized phase II study of three doses of oral TAS-108 in postmenopausal patients with metastatic breast cancer
- PMID: 22676245
- PMCID: PMC7659257
- DOI: 10.1111/j.1349-7006.2012.02354.x
Randomized phase II study of three doses of oral TAS-108 in postmenopausal patients with metastatic breast cancer
Abstract
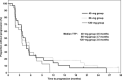
This randomized phase II study was intended to identify the optimal dose of TAS-108, a novel steroidal antiestrogen, for the treatment of breast cancer in postmenopausal Japanese women. The potential clinical effects of TAS-108 on the uterus, bone, serum lipids, and hormones were also investigated. Postmenopausal women with hormone receptor-positive metastatic breast cancer who had previously received one or two endocrine therapies were randomly assigned to one of the three possible dose levels of TAS-108 (40, 80 or 120 mg/day). Oral TAS-108 was given daily, and the efficacy and safety of the three doses were evaluated. A total of 97 patients (33, 32, and 32 in the 40-, 80-, and 120-mg groups, respectively) were treated with TAS-108. The clinical benefit rate was 30.3% for the 40-mg, 25.0% for the 80-mg, and 25.0% for the 120-mg group. The 40-mg group achieved the prespecified target threshold. TAS-108 at all dose levels was well tolerated and appeared to have no harmful effects in terms of the variables examined in this study. We conclude that the optimal dose of TAS-108 among the three doses is 40 mg, once daily, for further studies. JAPIC Clinical Trials Information number: Japic CTI - 121754.
© 2012 Japanese Cancer Association.
Figures
Similar articles
-
Evaluation of the safety and tolerability of oral TAS-108 in postmenopausal patients with metastatic breast cancer.Ann Oncol. 2009 May;20(5):868-73. doi: 10.1093/annonc/mdn714. Epub 2009 Jan 15. Ann Oncol. 2009. PMID: 19150935 Clinical Trial.
-
Randomized double-blind phase 2 trial of 3 doses of TAS-108 in patients with advanced or metastatic postmenopausal breast cancer.Cancer. 2012 Jul 1;118(13):3244-53. doi: 10.1002/cncr.26419. Epub 2011 Nov 1. Cancer. 2012. PMID: 22045595 Clinical Trial.
-
[Fulvestrant in heavily pretreated postmenopausal women with advanced breast cancer].Med Clin (Barc). 2009 Sep 19;133(10):371-4. doi: 10.1016/j.medcli.2008.11.029. Epub 2009 Apr 1. Med Clin (Barc). 2009. PMID: 19339025 Spanish.
-
Fulvestrant: a review of its use in hormone receptor-positive metastatic breast cancer in postmenopausal women with disease progression following antiestrogen therapy.Drugs. 2004;64(6):633-48. doi: 10.2165/00003495-200464060-00009. Drugs. 2004. PMID: 15018596 Review.
-
Fulvestrant: a review of its use in the management of hormone receptor-positive metastatic breast cancer in postmenopausal women.Drugs. 2011 Feb 12;71(3):363-80. doi: 10.2165/11204810-000000000-00000. Drugs. 2011. PMID: 21319872 Review.
Cited by
-
Development of new estrogen receptor-targeting therapeutic agents for tamoxifen-resistant breast cancer.Future Med Chem. 2013 Jun;5(9):1023-35. doi: 10.4155/fmc.13.63. Future Med Chem. 2013. PMID: 23734685 Free PMC article. Review.
-
SR-16234, a Novel Selective Estrogen Receptor Modulator for Pain Symptoms with Endometriosis: An Open-label Clinical Trial.Yonago Acta Med. 2018 Feb 5;60(4):227-233. doi: 10.24563/yam.2017.12.003. eCollection 2017 Dec. Yonago Acta Med. 2018. PMID: 29434492 Free PMC article.
-
Ginkgetin induces cell death in breast cancer cells via downregulation of the estrogen receptor.Oncol Lett. 2017 Oct;14(4):5027-5033. doi: 10.3892/ol.2017.6742. Epub 2017 Aug 10. Oncol Lett. 2017. PMID: 29085516 Free PMC article.
References
-
- Mouridsen H, Gershanovich M, Sun Y et al Superior efficacy of letrozole versus tamoxifen as first‐line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol 2001; 19: 2596–606. - PubMed
-
- Nabholtz JM, Buzdar A, Pollak M et al Anastrozole is superior to tamoxifen as first‐line therapy for advanced breast carcinoma in postmenopausal women: results of a North American multicenter randomized trial. J Clin Oncol 2000; 18: 3758–67. - PubMed
-
- Viale G, Giobbie‐Hurder A, Regan MM et al Prognostic and predictive value of centrally reviewed Ki‐67 labeling index in postmenopausal women with endocrine‐responsibe breast cancer: results from Breast International Group Trial 1‐98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol 2008; 26: 5569–75. - PMC - PubMed
-
- Bertelli G, Paridaens R. Optimal sequence of hormonotherapy in advanced breast cancer. Curr Opin Oncol 2006; 18: 572–7. - PubMed
-
- Nayfield SG, Karp JE, Ford LG, Dorr FA, Kramer BS. Potential role of tamoxifen in prevention of breast cancer. J Natl Cancer Inst 1991; 83: 1450–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical