The effect of ultrapro or prolene mesh on postoperative pain and well-being following endoscopic Totally Extraperitoneal (TEP) hernia repair (TULP): study protocol for a randomized controlled trial
- PMID: 22676248
- PMCID: PMC3404916
- DOI: 10.1186/1745-6215-13-76
The effect of ultrapro or prolene mesh on postoperative pain and well-being following endoscopic Totally Extraperitoneal (TEP) hernia repair (TULP): study protocol for a randomized controlled trial
Abstract
Background: The purpose of this study was to describe the rationale and design of a randomized controlled trial analyzing the effects of mesh type (Ultrapro versus Prolene mesh) on postoperative pain and well-being following an endoscopic Totally Extraperitoneal (TEP) repair for inguinal hernias (short: TULP trial).
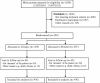
Methods and design: The TULP trial is a prospective, two arm, double blind, randomized controlled trial to assess chronic postoperative pain and quality of life following implantation of a lightweight (Ultrapro) and heavyweight (Prolene) mesh in endoscopic TEP hernia repair. The setting is a high-volume single center hospital, specializing in TEP hernia repair. All patients are operated on by one of four surgeons. Adult male patients (≥18 years of age) with primary, reducible, unilateral inguinal hernias and no contraindications for TEP repair are eligible for inclusion in the study. The primary outcome is substantial chronic postoperative pain, defined as moderate to severe pain persisting ≥ 3 months postoperatively (Numerical Rating Scale, NRS 4-10). Secondary endpoints are the individual development of pain until three years after the TEP procedure, the quality of life (QoL), recurrence rate, patient satisfaction and complications.
Discussion: Large prospective randomized controlled studies with a long follow-up evaluating the incidence of chronic postoperative pain following implantation of lightweight and heavyweight mesh in endoscopic (TEP) hernia repair are limited. By studying the presence of pain and quality of life, but also complications and recurrences in a large patient population, a complete efficiency and feasibility assessment of both mesh types in TEP hernia repair will be performed.
Trial registration: The TULP study is registered in the Dutch Trial Register (NTR2131).
Figures
Similar articles
-
Three-month results of the effect of Ultrapro or Prolene mesh on post-operative pain and well-being following endoscopic totally extraperitoneal hernia repair (TULP trial).Surg Endosc. 2015 Nov;29(11):3171-8. doi: 10.1007/s00464-014-4049-x. Epub 2015 Jan 1. Surg Endosc. 2015. PMID: 25552236 Clinical Trial.
-
Higher Recurrence Rate After Endoscopic Totally Extraperitoneal (TEP) Inguinal Hernia Repair With Ultrapro Lightweight Mesh: 5-Year Results of a Randomized Controlled Trial (TULP-trial).Ann Surg. 2018 Aug;268(2):241-246. doi: 10.1097/SLA.0000000000002649. Ann Surg. 2018. PMID: 29303810 Clinical Trial.
-
Long-term Results of a Randomized Double-blinded Prospective Trial of a Lightweight (Ultrapro) Versus a Heavyweight Mesh (Prolene) in Laparoscopic Total Extraperitoneal Inguinal Hernia Repair (TULP-trial).Ann Surg. 2016 May;263(5):862-6. doi: 10.1097/SLA.0000000000001579. Ann Surg. 2016. PMID: 26779980 Clinical Trial.
-
Lightweight mesh is recommended in open inguinal (Lichtenstein) hernia repair: A systematic review and meta-analysis.Surgery. 2020 Mar;167(3):581-589. doi: 10.1016/j.surg.2019.08.021. Epub 2019 Oct 28. Surgery. 2020. PMID: 31672519
-
Meta-Analysis of Randomized Controlled Trials Comparing Lightweight and Heavyweight Mesh for Laparoscopic Total Extraperitoneal Inguinal Hernia Repair.Am Surg. 2019 Jun 1;85(6):620-624. Am Surg. 2019. PMID: 31267903 Review.
Cited by
-
Is young age a risk factor for chronic postoperative inguinal pain after endoscopic totally extraperitoneal (TEP) repair?Hernia. 2019 Dec;23(6):1053-1059. doi: 10.1007/s10029-019-01882-3. Epub 2019 Jan 17. Hernia. 2019. PMID: 30652223
-
Three-month results of the effect of Ultrapro or Prolene mesh on post-operative pain and well-being following endoscopic totally extraperitoneal hernia repair (TULP trial).Surg Endosc. 2015 Nov;29(11):3171-8. doi: 10.1007/s00464-014-4049-x. Epub 2015 Jan 1. Surg Endosc. 2015. PMID: 25552236 Clinical Trial.
References
-
- Simons MP, de Lange D, Beets GL, van Geldere D, Heij HA, P.M.N.Y.H Go. Richtlijn Liesbreuk van de Nederlandse Verenigning voor Heelkunde. http://www.ntvg.nl/publicatie/richtlijn-liesbreuk-van-de-nederlandse-/vo.... - PubMed
-
- Simons MP, Aufenacker T, Bay-Nielsen M, Bouillot JL, Campanelli G, Conze J, de Lange D, Fortelny R, Heikinnen T, Kingsnorth A, Kukleta J, Morales-Conde S, Nordin P, Schumpelick V, Smedberg S, Smietanski M, Weber G, Miserez M. European Hernia Society guidelines on the treatment of inguinal hernia in adult patients. Hernia. 2009;13:343–403. doi: 10.1007/s10029-009-0529-7. - DOI - PMC - PubMed
-
- Bozuk M, Schuster R, Stewart D, Hicks K, Greaney G, Waxman K. Disability and chronic pain after open mesh and laparoscopic inguinal hernia repair. Am Surg. 2003;69:839–841. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical