Common brain activations for painful and non-painful aversive stimuli
- PMID: 22676259
- PMCID: PMC3464596
- DOI: 10.1186/1471-2202-13-60
Common brain activations for painful and non-painful aversive stimuli
Abstract
Background: Identification of potentially harmful stimuli is necessary for the well-being and self-preservation of all organisms. However, the neural substrates involved in the processing of aversive stimuli are not well understood. For instance, painful and non-painful aversive stimuli are largely thought to activate different neural networks. However, it is presently unclear whether there is a common aversion-related network of brain regions responsible for the basic processing of aversive stimuli. To help clarify this issue, this report used a cross-species translational approach in humans (i.e. meta-analysis) and rodents (i.e. systematic review of functional neuroanatomy).
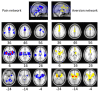
Results: Animal and human data combined to show a core aversion-related network, consisting of similar cortical (i.e. MCC, PCC, AI, DMPFC, RTG, SMA, VLOFC; see results section or abbreviation section for full names) and subcortical (i.e. Amyg, BNST, DS, Hab, Hipp/Parahipp, Hyp, NAc, NTS, PAG, PBN, raphe, septal nuclei, Thal, LC, midbrain) regions. In addition, a number of regions appeared to be more involved in pain-related (e.g. sensory cortex) or non-pain-related (e.g. amygdala) aversive processing.
Conclusions: This investigation suggests that aversive processing, at the most basic level, relies on similar neural substrates, and that differential responses may be due, in part, to the recruitment of additional structures as well as the spatio-temporal dynamic activity of the network. This network perspective may provide a clearer understanding of why components of this circuit appear dysfunctional in some psychiatric and pain-related disorders.
Figures


Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Pain management for women in labour: an overview of systematic reviews.Cochrane Database Syst Rev. 2012 Mar 14;2012(3):CD009234. doi: 10.1002/14651858.CD009234.pub2. Cochrane Database Syst Rev. 2012. PMID: 22419342 Free PMC article.
Cited by
-
Perceptual Sensitivity and Response to Strong Stimuli Are Related.Front Psychol. 2017 Sep 22;8:1642. doi: 10.3389/fpsyg.2017.01642. eCollection 2017. Front Psychol. 2017. PMID: 29018377 Free PMC article.
-
Affective Circuitry Alterations in Patients with Trigeminal Neuralgia.Front Neuroanat. 2017 Sep 5;11:73. doi: 10.3389/fnana.2017.00073. eCollection 2017. Front Neuroanat. 2017. PMID: 28928638 Free PMC article.
-
Novel method for functional brain imaging in awake minimally restrained rats.J Neurophysiol. 2016 Jul 1;116(1):61-80. doi: 10.1152/jn.01078.2015. Epub 2016 Apr 6. J Neurophysiol. 2016. PMID: 27052584 Free PMC article.
-
Resting state hypothalamic and dorsomedial prefrontal cortical connectivity of the periaqueductal gray in cocaine addiction.Addict Biol. 2021 Jul;26(4):e12989. doi: 10.1111/adb.12989. Epub 2020 Dec 9. Addict Biol. 2021. PMID: 33300238 Free PMC article.
-
Anticipating control over aversive stimuli is mediated by the medial prefrontal cortex: An fMRI study with healthy adults.Hum Brain Mapp. 2021 Sep;42(13):4327-4335. doi: 10.1002/hbm.25549. Epub 2021 Jun 9. Hum Brain Mapp. 2021. PMID: 34105855 Free PMC article.
References
-
- Ardiel EL, Rankin CH. An elegant mind: learning and memory in Caenorhabditis elegans. Learn Mem. 2010;17(4):191–201. - PubMed
-
- Glanzman DL. Associative learning: Hebbian flies. Curr Biol. 2005;15(11):R416–419. - PubMed
-
- Seymour B, Singer T, Dolan R. The neurobiology of punishment. Nat Rev Neurosci. 2007;8(4):300–311. - PubMed
-
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5(6):483–494. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

